Glass Electrode for pH Measurement
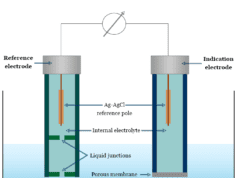
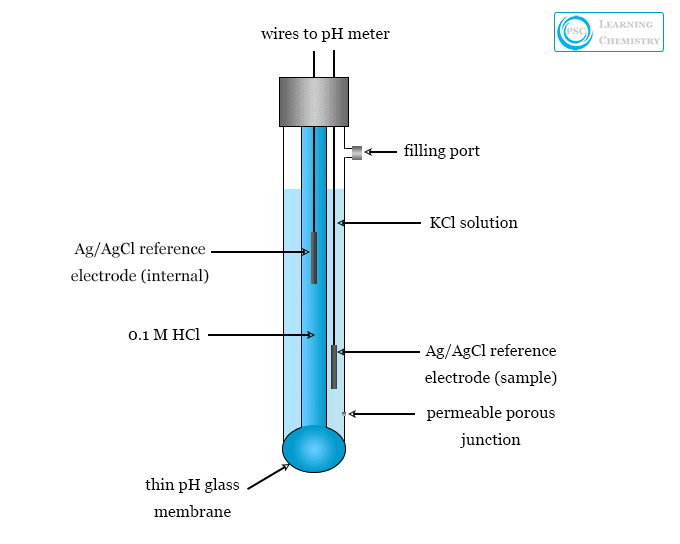
Glass electrode is a type of ion selective electrode used mainly for the measurement of the pH of a solution. A glass electrode consists of a glass tube terminating in a thin size membrane. The modern pH probe in the glass electrodes contains a hydrogen ion sensitive glass bulb. The function of the glass electrode is based on the fact that when a thin glass membrane separates two solutions, a potential is developed across the membrane. The magnitude of potential depends mainly on the pH of the solutions. A modern pH meter has a glass electrode and a reference electrode or a combination electrode. The electrodes or probes are inserted into the solution which we want to test.
The electrode potential can be used for the pH mesurement of the solution. The half-cell reaction of the glass electrode is represented as,
H+ (unknown) | glass membrane | 0.1 N HCl | AgCl (s) – Ag
Construction and Working of Glass Electrode
The glass electrode is constructed from a special soft glass of high electrical conductance. It consists of a bulb that contains a solution of constant hydrogen ion concentration.
- Usually, the solution contains 0.1 N hydrochloric acid (HCl) in which a silver/silver chloride electrode is dipped. The blub is then placed in a solution of unknown pH.
- Instead of using Ag/AgCl electrode dipped in 0.1 N HCl, a platinum wire can be inserted in a pH 4.0 buffer solution containing a small quantity of quinhydrone.
The potential of the electrode is similar to that of a standard hydrogen electrode,
Eglass = E0glass + 0.059 log aH+ at 25 °C
or, Eglass = E0glass + 0.059 log pH
Here E0glass is called asymmetric potential and the value is small. This type of potential is developed across the membrane due to internal strain. It is the characteristic constant for each glass electrode.
Glass Membranes
Almost all commercial pH-sensitive glasses in glass membrane electrodes respond to single-charged ions such as H+, Na+, and Ag+ ions. pH-electrode is most common in that class. Few chalcogenide glass membranes are sensitive to double-charged ions such as Pb2+, and Cd2+ ions.
Most commercial glass membranes are manufactured based on a tetrahedral network of silicon dioxide (SiO2) by adding oxides of sodium, potassium, lithium, aluminum, boron, or calcium.
Asymmetric Potential in Glass Electrode
To determine the value of E0glass, the electrode is dipped into a solution of known pH buffer solution and coupled with a secondary reference electrode such as a standard calomel electrode through a salt bridge. The entire assembly which constitutes a cell may be represented below
− Ag – AgCl (s) | 0.1 N HCl | glass membrane | buffer solution of known pH | saturated calomel +
The emf of the cell, E = Ecalomel – Eglass.
Here Ecalomel = 0.2415 volt at 25 °C.
Therefore, E = 0.2415 − E0 glass + 0.059 pH.
The potential E is determined by the electronic voltameter since an ordinary potentiometer cannot be used due to the high resistance of the glass membrane.
pH Measurement

The emf of the call at 25 °C
E = 0.2415 − E0 glass + 0.059 pH
The above glass electrode setup can be used for the measurement of the pH of an unknown solution by the known value of E0glass. The working principle of the pH meter is also based on the above glass electrode setup.
Otherwise, cell potential E1 is measured with a buffer of known pH1. Again the cell potential E is determined with a solution of unknown pH value.
If we used the same cell setup in both the cases,
ΔE = E − E1 = 0.059 (pH − pH1)
pH Glass Electrode
The pH electrode is an example of an ion selective glass electrode. It is sensitive to hydrogen ions which are commonly used for pH measurements. A modern pH meter is a combination of glass and reference electrodes in one body. Many specialized types of ion sensitive glass electrodes are used for the determination of the concentration of metal ions such as lithium, sodium, ammonium, etc.
Advantages
The common advantages of the application of glass electrodes in pH measurement are,
- The ion selective glass electrode gives a fairly accurate measurement of pH within an error of ± 0.05.
- The equilibrium of the electrode is quickly attained. It cannot be influenced by any gas, oxidizing, or reducing agents.
- The range of pH which can be measured is 2 to 9. However, new glasses have been developed with which the pH range can be extended up to 13 or 14.
- Glass electrodes are used for a long time for pH measurement if carefully handled and stored in distilled water.
Application
pH measurement by glass electrodes is used in many chemical and industrial analyses. The main applications are
- Control of industrial processes;
- Analysis of foods and cosmetics;
- Clinical analysis;
- Environmental analysis;
- Microelectrode measurements;
- Measurement of soil pH level by glass electrode is an important process to observe soil acidity.