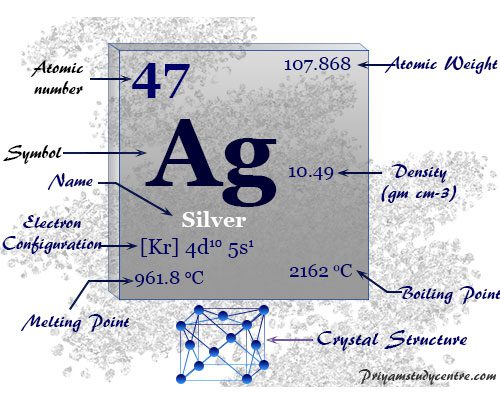
Silver Element (Ag)
Silver is the shiny white chemical element or lustrous transition metal of Group 11 or IB in the periodic table with atomic number 47 and the symbol Ag. Chemically, silver metal is a quite unreactive chemical element with a good conductor of electricity. The atomic states of copper, silver, and gold contain ns1 (n−1)d10 outer electron configuration or filled d-orbital. They are considered transition metals because, in +2 or +3 oxidation number or state, they possess incompletely filled d-orbital. Silver element generally forms an FCC crystal lattice with characteristic silvery-white colour. It has been used since the ancient age for making jewelry and coinage due to its decorative beauty and unreactive properties. The name silver comes from Assyrian serpu or Gothic silbur meaning white and from the Latin name, Argentum means shiny white (Greek latter Argos).
Where is Silver Found?
The relative occurrence of metal in the earth’s crust is 0.08 ppm while that of gold is 0.004 ppm.
The metal Ag is widely distributed in nature as sulfide ore like a silver glance or argentite (Ag2S). Horn silver (AgCl) is also found in some minerals in Chile and New South Wales formed by the action of salt water. It is now largely discovered as a byproduct of the extraction of copper and lead.
Silver element is found in various countries such as the United States, Canada, Peru, Mexico, Bolivia, and New South Wales. In India, the metal Ag is obtained generally from Kolar gold mines produced from the smelting of Zawar lead ores.
Properties of Silver
In learning chemistry, the metal Ag dissolved in water to the extent of 0.07 mg liter−1 in the presence of dissolved oxygen. It is attacked by atmospheric sulfur compounds, mainly hydrogen sulfide (H2S).
| Silver |
|||
| Symbol | Ag | ||
| Discovery | Approx 3000BC | ||
| Name derived from | Assyrian serpu or Gothic silbur meaning white and from the Latin name, Argentum means shiny white (Greek latter Argos) | ||
| Common isotope | 47Ag107 | ||
| Oxidation states | 2, 1 | ||
| CAS number | 7440-22-4 | ||
| Periodic properties |
|||
| Atomic number | 47 | ||
| Atomic weight | 107.868 | ||
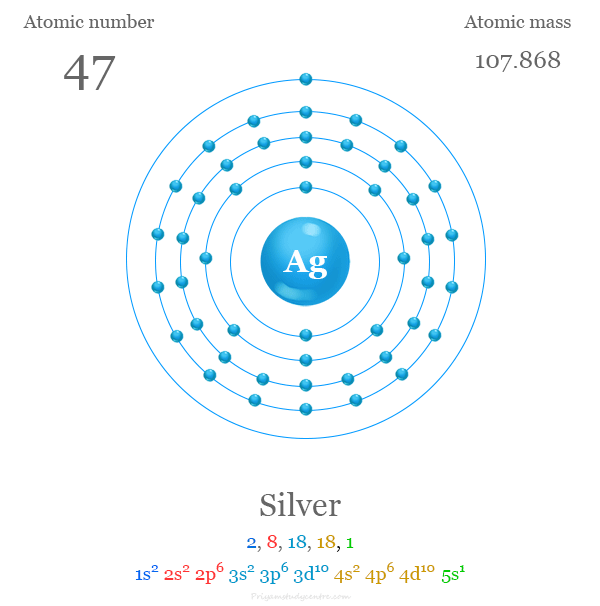
| Electron per cell | 2, 8, 18, 18, 1 | ||
| Electronic Configuration | [Kr] 4d10 5S1 | ||
| Group | 11 | ||
| Period | 5 | ||
| Block | d-block | ||
| Physical properties |
|||
| State at 20 °C | Solid | ||
| Melting point | 961.8 °C or 1763.2 °F | ||
| Boiling point | 2162 °C or 3924 °F | ||
| Density | 10.49 g/cm3 | ||
| Electrical resistivity | 15.87 nΩ m | ||
| Molar heat capacity | 25.350 J mol−1 K−1 | ||
| Crystal structure | Face-centred cubic (fcc) | ||
| Atomic properties |
|||
| Atomic radius (non-bonded) | 2.11 Å | ||
| Covalent radius | 1.36 Å | ||
| Electronegativity | 1.93 (Pauling scale) | ||
| Electron affinity | 125.62 kJ mol−1 | ||
| Ionization energy (kJ mol−1) | 1st | 2nd | 3rd |
| 730.99 | 2072.26 | 3360.58 | |
Electron Configuration
The 47 electrons of the silver atom are distributed in different energy levels to show the following electronic configuration given below the picture,
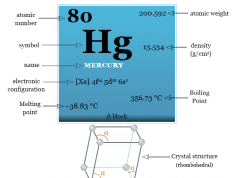
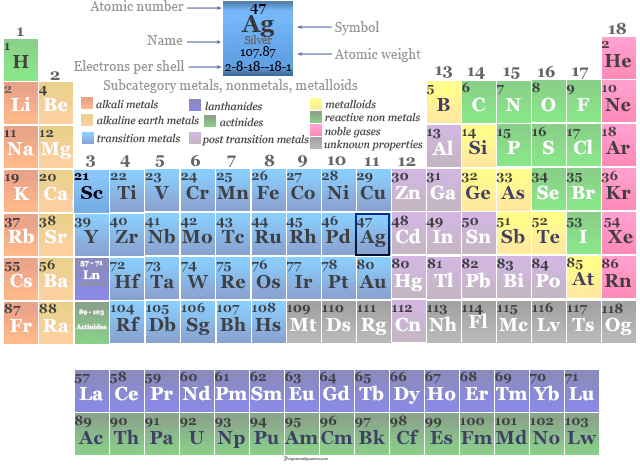
Silver in the Periodic Table
The element Ag is placed in group 11 in the periodic table with d-block elements.
Production of Silver
- Mostly, the elemental silver was obtained as a byproduct for the production of copper, lead, and zinc by collecting anode slime or mud.
- The anode slime or mud is heated with dilute sulfuric acid in a stream of air to dissolve some metals.
- The remaining anode slug was heated with lime and silica to remove most of the base metal as a slug.
- Finally, Ag metal containing materials are dissolved in dilute nitric acid.
- Electrolysis of AgNO3 solution gives a pure form of silver metal at the cathode.
Extraction of Silver from Ore
- The element Ag is extracted from its important ores by leaching the finely powdered ore with dilute (0.4 percent) sodium cyanide solution.
- The sludge is ignited well with air. The Na2S is oxidized by aeration, otherwise, it would tend to reverse the reaction.
- The sludge is removed by filtration and the filtrate silver is precipitated by zinc or aluminum.
- The precipitate is washed and melted with a flux of nitrate to remove the excess zinc.
Chemical Compounds
The physical and chemical properties of silver are similar to the two vertical neighbors copper and gold on the periodic table. The higher effective nuclear charge or ionic potential and large shielding electron suggest that the covalent bonding of Ag and Au compounds are stable in +1 and +2 states.
The +1 state is the most common oxidation state of silver which offers oxides, sulfides, halides, and a number of oxoacids salts.
The salts are primarily ionic and quite soluble in water but Ag2SO4 and CH3COOAg are sparingly soluble in water. The halide (except AgF) is formed by covalent chemical bonding.
Oxidation State
The +1 oxidation state of silver is the most common and stable because the first ionization energy is lowest for the element Ag. The sum of the first and second ionization is the lowest for Cu. However, the sum of the first, second, and third ionization energy is the lowest for Au atoms.
The above fact reflects that the chemistry of Ag metal is predominant in the +1 oxidation state. However, copper and gold chemistry predominates in the +2 and +3 oxidation states.
The facts are also described by hydration energy.
- The hydration energy of the copper (II) ion is much higher than the copper (I) ion which overcomes the second ionization of Cu in an aqueous medium.
- Due to higher ionic radii of Ag, the hydration energy cannot stabilize Ag (II) ion over Ag (I).
- For gold atoms, atomization, ionization, and hydration energy favor the formation of Au (III) in an aqueous medium.
Silver Oxide
The dark brown silver oxide (formula Ag2O) is obtained by adding alkali to an aqueous Ag+ ion. It is more soluble in alkali than pure water owing to the formation of Ag(OH)2−.
Ag2O dissolves in ammonia to produce [Ag(NH3)2]OH. When the solution is exposed to air, it deposits the black explosive silver nitride (Ag3N). It is called fulminating silver.
Moist Ag2O is used as a mild oxidizing agent for organic compounds.
A black oxide like AgO is formed by oxidation of Ag2O with ozone molecule or by electrolysis 2M AgNO3 solution.
Silver (II) Compounds
Ag (II) compounds are obtained by direct oxidation or disproportionate of the chemical element. They are extremely unfavorable due to the low heat of hydration of Ag+2 ions.
AgF2 is the only known silver halide obtained by the action of fluorine on AgF or Ag at 250 °C temperature. It is used as a fluorinating agent.
Silver (III) Compounds
Compounds of Ag (III) are very few, Ag2O3, Ag(OH)4−, KAgF4, and Cs2KAgF6 are the common examples of the +3 oxidation state of the element silver.
The formal oxidation number +1/2 is present in the yellow-green solid Ag2F.
Uses of Silver
- Silver element (Ag) and its compounds are mostly used in photography, in mirrors or plates, in silverware, jewelry, dentistry, and coins.
- The element Ag is also used in high-capacity electrical cells (Ag-Zn, Ag-Cd).
- Copper serves to increase the hardness of alloy to improve the wearing qualities.
- The photographic light sensitivity plate consists of an emulsion of fine-grain silver halides like bromide, chloride, or iodide (diameter < 1 μm) on a transparent medium like glass or celluloid.
- The halide, usually AgBr with some amount of AgCl and organic dyes but AgI is used in very fast films. Organic dyes act as a photosensitizer to help light absorption over the entire visible band.
- Silver plating in chemistry is done at the cathode of the electrolytic cell. Therefore, a piece of pure Ag element acts as an anode with the electrolytic solution sodium dicyanoargentate, Na[Ag(CN)2].
Detection of Silver Ion
Silver salts in an aqueous solution produce a curdy white precipitate of AgCl with chlorine ions. The precipitate is insoluble in nitric acid but readily dissolved in aqueous ammonia.
The silver ion (Ag+) also forms a red precipice (Ag2CrO4) with potassium dichromate solution which is insoluble in acetic acid.
All the silver compounds produce a lustrous white malleable bed when heated with sodium carbonate on charcoal. The bead may be dissolved in nitric acid and tested with hydrogen chloride (HCl).
Gravimetric Analysis
In learning chemistry or chemical science, silver may be estimated gravimetrically as chloride or by electrodeposition (coulometer), volumetrically.
- In Mohrs’s method, a known volume of standard sodium chloride solution is titrated with AgNO3 solution using potassium chromate as an indicator.
- In Volhard’s method, the silver nitrate (AgNO3) is titrated by standard potassium or ammonium thiocyanate using ferric alum as an indicator.
The silver ion (Ag+) forms a white precipitate of AgSCN but when the concentration falls below the solubility, the blood-red color of thiocyanate ions is produced as a ferric ion.