Bohr Model of Hydrogen Atom
Bohr Model of hydrogen atom was adopted by Neils Bohr in 1913 for the explanation of the Rutherford model and the atomic energy levels of the hydrogen spectrum.
According to classical mechanics, when a charged electron is subjected to acceleration, it emits electromagnetic radiation and hits the nucleus of an atom.
According to Rutherford’s model, the electron loses energy continuously and the observed spectra should be continuous spectra. However, the actual electromagnetic spectrum of the hydrogen atom consists of well-defined lines with definite frequencies or wavelengths.
For an explanation of the atomic spectrum, Bohr adopted the Rutherford nuclear model for the hydrogen atom or one electronic system in which an electron revolves around the nucleus. The Bohr atomic model of the hydrogen atom is similar to that of the solar system of our planet.
Bohr Model of Hydrogen Atom Postulates
To study chemistry or physics, Bohr’s model of a hydrogen atom is based on several postulates. The postulates may include,
- An atom or hydrogen atom possesses several stable circular orbitals in which an electron can stay.
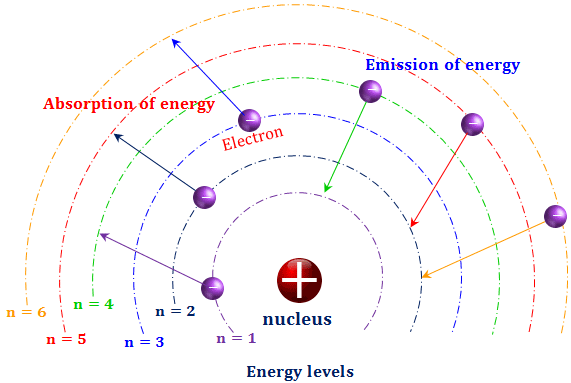
- An electron stays in a particular orbit where no emission or absorption of energy occurs.
- An electron can jump from one orbit to another higher energy orbit on the absorption of energy but from one orbit to another lower energy orbit with the emission of energy.
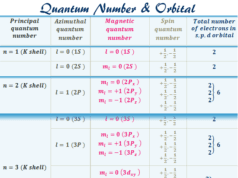
- The angular momentum of an electron moving in an orbit is an integral multiple of h/2π. This integral multiple is known as the principal quantum energy level of the hydrogen atom.
From the above postulate, mvr = nh/2π, where m = mass of an electron, v = tangential velocity of an electron, r = radius of Bohr energy levels, and h = 6.627 × 10−27 erg sec.
Hydrogen Energy Levels
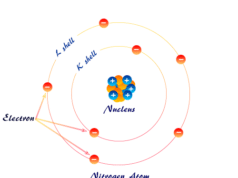
An electron stays in a particular orbit where no emission or absorption of energy occurs. These orbits are called the energy levels of the hydrogen atom.
The energy levels are designated by numbers 1, 2, 3, … or capital letters, K, L, M, … starting from the nucleus of the atom. Therefore the energy associated with certain hydrogen energy levels increases with the increase of its distance from the nucleus.
If E1, E2, E3 … denotes the energy levels of 1 (K-shell), 2 (L-shell), 3 (M-shell) …, then the order of energy levels is E1ㄑE2ㄑE3ㄑ…
Electron Attraction to the Nucleus
Let the nucleus have a mass of m′ and the electron has a mass of m, the radius of the circular orbit = r, and the linear velocity of the electron = v.
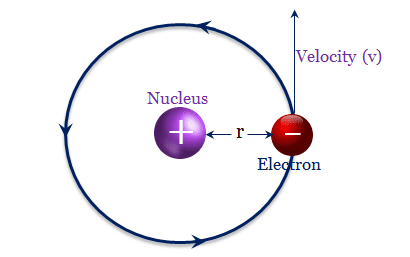
On the revolving electron in the hydrogen atom, two types of forces act,
- Centrifugal force: The centrifugal force = mv2/r
- Electric force of attraction: Electric force of attraction given from Coulomb’s law between two opposite charges = e2/r2
Centrifugal force and electric force acted in the opposite direction. The electron may keep on revolving in hydrogen atom energy levels if these two forces act in the opposite direction and must balance each other. Therefore, mv2 = e2/r.
Radius of Hydrogen Atom
From Niels Bohr’s atomic theory, the angular momentum of the electron is an integral multiple of h/2π.
∴ mvr = nh/2π
or, v = nh/2πmr
where mvr = angular momentum
n = principal quantum number =1,2,3, …, ∞
If we put the value of velocity (v) on mv2 = e2/r and rearrange the equation,
The radius of the hydrogen atom,
r = n2h2/4π2me2
Radius of First Orbit of Hydrogen Atom
From the above equation, we can easily find out the radius of the permitted energy levels in terms of the quantum number of the hydrogen atom.
When n = 1, the radius of the first stationary orbit of hydrogen,
r1 = 0.529 × 10−8 cm
= 0.529 Å
The relation between the first Bohr orbit and the nth orbit of the hydrogen atom,
rn = n2 × r1 = n2 × 0.529 Å
Velocity of Electron in Bohr Model
From the principle of quantization to the revolving electron in the hydrogen atom, mvr = nh/2π.
When we put the values of r = n2h2/4π2me2 in equation mvr = nh/2π and solve the equation, the velocity in the Bohr electron,
v = 2πe2/nh
From the above formula, the velocity of the second orbit will be one-half of the first orbit, one-third of the first orbit, and so on.
Energy of Electron in Bohr Model
The energy of an electron moving in one particular energy level of the hydrogen atom in the Bohr models can be calculated by the total energy or the sum of the kinetic energy and the potential energy of an electron.
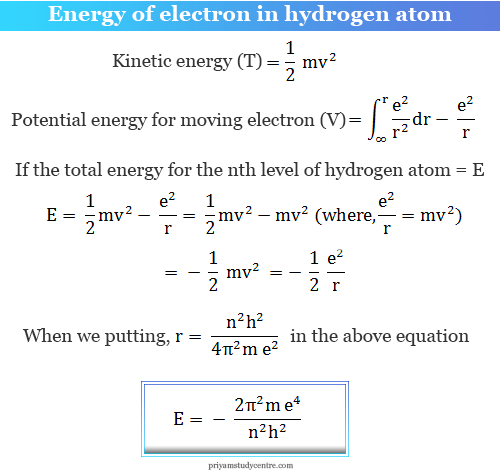
Putting the values of π, m, e, and h in the above expression of E, we get the numerical values of E1.
E1 = −21.79 × 10−12 erg
= −13.6 eV
= −21.79 × 10−19 Joule
Or, E1 = −313.6 kcal
In learning chemistry,
En = E1/n2
where E1 = energy of the first orbit of the hydrogen atom in the Bohr model.
As the principal quantum number increases the energy becomes less negative and hence the Bohr system becomes less stable. Because with increasing n, r also increases and orbit makes less stable.
If the energies associated with 1st, 2nd, 3rd,.., nth orbits in the hydrogen atom be E1, E2, E3 … En, according to the energy equation of the Bohr model,
E1 ㄑ E2 ㄑ E3 ㄑ…ㄑEn
Energy Equation in Bohr Model
The energy of the moving electron in the various energy levels is obtained by putting the values of n in the Bohr energy equation. The energy E1, E2, E3, E4, E5 in the Bohr model of the hydrogen atom,
- E2 = −13.6/22 = −3.4 eV
- E3 = −13.6/32 = −1.51 eV
- E2 = −13.6/42 eV = −0.85 eV
- E2 = −13.6/52 eV = −0.54 eV
Bohr Model Practice Problems
Problem: Calculate the radius of the second orbit of the hydrogen atom if the radius of the first orbit of hydrogen = 0.529 Å.
Solution: The radius of the second orbit of the hydrogen atom (r2) = n2 × r1 = 2.12 Å.
Problem: Calculate the velocity of the hydrogen electron in the first and third energy levels of the Bohr model. How to calculate the number of rotations of an electron per second in the third energy level in the Bohr model?
Solution: The velocity of an electron nth level of the hydrogen atom,
vn = 2πe2/nh
where n = 1, 2, 3, …, ∞
From the above relation, the velocity of an electron in the first energy level,
v1 = 2.188 × 108 cm sec−1
The velocity of the third energy level,
v3 = 7.30 × 107 cm sec−1
The radius of the third energy level,
r3 = 32 × 0.529 × 10−8 cm
Hence, the circumference of the third energy level,
= 2πr = 2 × 3.14 × 0.529 × 10−8 cm
Therefore, the rotation of an electron per second in the third energy level,
= 2.44 × 1014 sec−1
Question: H, H+, He+, and Li+2 for which of the species Bohr’s model of the hydrogen atom is not applicable?
Answer: From the above species H, He+, Li+2 contain one electron but H+ ion has no electron.
Bohr model of the hydrogen atom is applicable to one electronic system in physics or chemistry but for H+ ion Bohr’s atomic model is not applicable.