Electron Configuration of Elements in Periodic Table
Electron configuration or electronic configuration or electronic structure of atoms or ions of s, p, d, and f block periodic table elements in chemistry is the arrangement or distribution of electrons in different orbitals or energy levels. In learning chemistry or chemical science, the electron holds the key to the chemical world and electronic configuration is useful to find the position of elements in the periodic table. The electronic configuration formula is also used to derive the chemical properties such as oxidizing and reducing, oxidation number, ionization energy, electron affinity, shielding effect, the polarity, acids bases properties, etc.
A reaction to reach chemical equilibrium is the change of electron configuration of reactant and product atoms. Hence the organic and inorganic chemical reactions are better understood by the electronic configuration of atoms of reacting elements.
How to Find Electronic Configuration of Elements?
To find the electron configuration formula first we write the order of electronic energy levels or s, p, d, and f orbitals. For example, the 3s orbital has lower energy than 3p orbitals which again has lower energy than the 3d level.
The modern periodic table classification like s, p, d, and f block elements is based on properties and general electron or electronic configuration of elements.
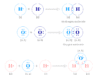
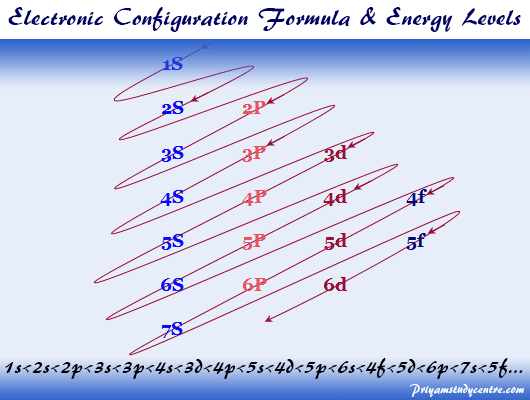
Electron Energy Levels Diagram
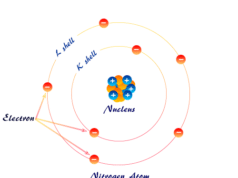
Energy levels are fixed distances where electrons are rotating around the nucleus with definite energy. The energy associated with a certain energy level increases with the increase of its distance from the nucleus.
The hydrogen atom contains only one electron in 1s hydrogen energy levels with electronic configuration 1s1.
But difficult for readers to remember the electron energy levels diagram for many electronic configurations. Therefore, the trivial way but most convenient way to remember these electronic energy levels is given above the diagram.
- The different electron orbitals originating from the same electronic energy levels are written in horizontal lines.
- Now inclined parallel lines are drawn through the electronic orbitals according to the above picture.
- Filling up the different orbitals by the number of electrons will follow these lines’ configuration.
- According to the above diagram, energy levels ordering where electrons are distributed,
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f…
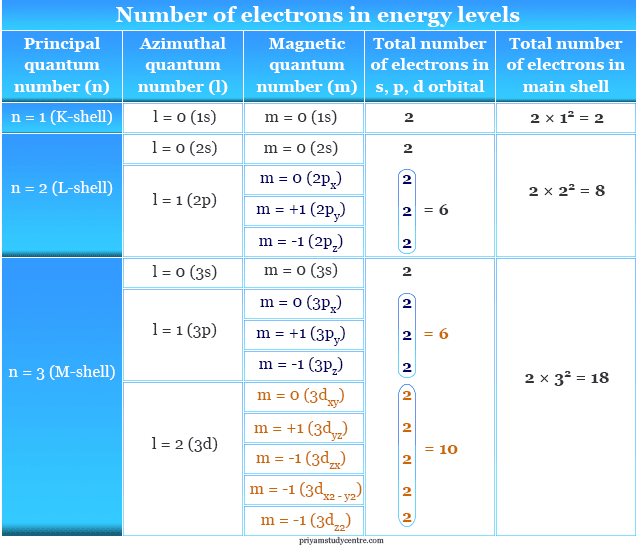
Electrons in Energy Levels
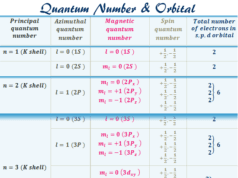
The electronic configuration or arrangement of atoms in different energy levels follows certain rules. The maximum number of electrons in the main energy levels = 2n2, where n = principal quantum number.
The maximum number of the electrons in sub-shells like s, p, d, and f orbitals = 2(2l + 1). Where l = 0, 1, 2, 3 for s, p, d, f orbitals. Therefore, s, p, d, and f orbitals contain a maximum of 2, 6, 10, and 14 electrons respectively.
Aufbau Principle
German scientist Aufbau expresses the building-up principle for the electron configuration process in different electronic orbitals of atoms.
According to the Aufbau principle, the electrons are filled up in order of energy. Therefore, the orbitals with the lowest energy filled up first while the highest energy orbital filled up in the end.
Hund’s Rule in Chemistry
According to Hund’s rule in chemistry, electrons are filling in the orbital with maximum spin multiplicity. Hence spin pairing occurs only when vacant orbitals of similar energy are not available for occupation.
The electron will tend to form maximum spin. Electrons with similar spin are configured first.
Electron Configuration and Periodic Table
The electronic configuration formula is very useful to derive some basic properties like the electromagnetic spectrum, chemical bonding, electric polarization, dipole moment, hydrogen bonding, etc.
Modern periodic tables are classified based on chemical behavior and the electronic configuration of elements. Therefore, the electron configuration formula of elements must be connected with the periodic table arrangement.
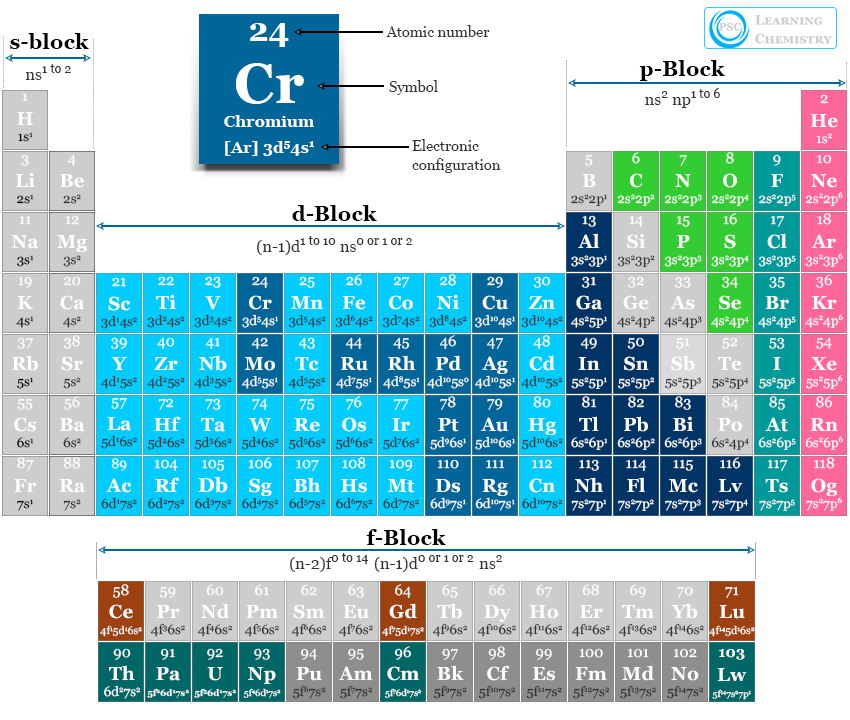
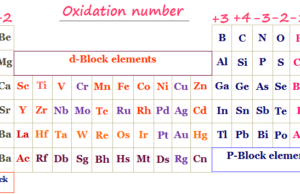
According to electronic configuration, we classify the periodic table elements into four blocks s, p, d, and f block elements.
Electronic Configuration of s Block Elements
For s block elements, the electron enters the ns orbitals and is progressively filled with atomic number.
Group 1 and group 2 belong to s block elements in the periodic table with general electron configuration ns1→2. Here n = the number of electronic shells or the number of periods in which the element stays.
Electronic Configuration of Alkali Metals
Group 1 or IA in the periodic table contains seven elements such as hydrogen, lithium, sodium, potassium, rubidium, cesium, and francium.
The general electronic configuration of valence electron = ns1, where n = 1 to 7. Due to the presence of one electron in the outer electronic structure, they possess very low ionization energy but very high electron affinity.
| Atomic number | Symbol | Name | Electronic configuration |
| 1 | H | Hydrogen | 1s1 |
| 3 | Li | Lithium | 1s2 2s1 |
| 11 | Na | Sodium | [Ne] 3s1 |
| 19 | K | Potassium | [Ar] 4s1 |
| 37 | Rb | Rubidium | [Kr] 5s1 |
| 55 | Cs | Cesium | [Xe] 6s1 |
| 87 | Fr | Francium | [Rn] 7s1 |
Electronic Configuration of Alkaline Earth Metals
The elements of Group 2 or IIA in the periodic table contain beryllium, magnesium, calcium, strontium, barium, and radium. They are also called alkaline earth metals.
The valence shell electronic configuration of group 2 elements or alkaline earth metals = ns2. Here n = 1 to 6.
| Atomic Number | Symbol | Name | Electronic configuration |
| 4 | Be | Beryllium | 1s2 2s2 |
| 12 | Mg | Magnesium | [Ne] 3s2 |
| 20 | Ca | Calcium | [Ar] 4s2 |
| 38 | Sr | Strontium | [Kr] 5s2 |
| 56 | Ba | Barium | [Xe] 6s2 |
| 88 | Ra | Radium | [Rn] 7s2 |
Electron Configuration of p Block Elements
The elements in which the p orbital is progressively filled by electrons are called p blocks in the periodic table. Helium whose electronic configuration is 1s2 but helium is a member of p block elements.
p block contains six groups from group 13 to group 18. The general electronic configuration formula to find the outer electron configuration of the p block element = ns2 np1→6.
Electronic Configuration of Group 13 Elements
Group 13 or IIIA contains five elements, boron, aluminum, gallium, indium, and thallium. The valence shell electron configuration of group 13 elements = ns2 np1.
| Atomic number | Symbol | Name | Electronic configuration |
| 5 | B | Boron | 1s2 2s2 2p1 |
| 13 | Al | Aluminum | [Ne] 3s2 3p1 |
| 31 | Ga | Galium | [Ar] 3d10 4s2 4p1 |
| 49 | In | Indium | [Kr] 4d10 5s2 5p1 |
| 81 | Tl | Thallium | [Xe] 4f14 5d10 6s2 6p1 |
| 113 | Nh | Nihonium | [Rn] 5f14 6d10 7s2 7p1 |
Electronic Configuration of Group 14 Elements
Carbon, silicon, germanium, tin, and lead in the periodic table belong to group 14 or IVA. The general electron configuration of group 14 elements = ns2 np2. Where n = 2 to 6.
| Atomic number | Symbol | Name | Electronic configuration |
| 6 | C | Carbon | 1s2 2s2 2p2 |
| 14 | Si | Silicon | [Ne] 3s2 3p2 |
| 32 | Ge | Germanium | [Ar] 3d10 4s2 4p2 |
| 50 | Sn | Tin | [Kr] 4d10 5s2 5p2 |
| 82 | Pb | Lead | [Xe] 4f14 5d10 6s2 6p2 |
| 114 | Fl | Flerovium | [Rn] 5f14 6d10 7s2 7p2 |
Electronic Configuration of Group 15 Elements
The five elements of Group 15 or VA contain nitrogen, phosphorus, arsenic, antimony, and bismuth. The general electron configuration of group 15 elements is written as ns2 np3. Here, n = 2 to 6.
| Atomic number | Symbol | Name | Electronic configuration |
| 7 | N | Nitrogen | 1s2 2s2 2p3 |
| 15 | P | Phosphorus | [Ne] 3s2 3p3 |
| 33 | As | Arsenic | [Ar] 3d10 4s2 4p3 |
| 51 | Sb | Antimony | [Kr] 4d10 5s2 5p3 |
| 83 | Bi | Bismuth | [Xe] 4f14 5d10 6s2 6p3 |
| 115 | Mc | Moscovium | [Rn] 5f14 6d10 7s2 7p3 |
Electronic Configuration of Group 16 Elements
Oxygen, sulfur, selenium, tellurium, and polonium in the periodic table belong to group 16 or VIA. The general electron configuration of group 16 elements is ns2 np4. Here, n = 2 to 6.
| Atomic number | Symbol | Name | Electronic configuration |
| 8 | O | Oxygen | 1s2 2s2 2p4 |
| 16 | S | Sulfur | [Ne] 3s2 3p4 |
| 34 | Se | Selenium | [Ar] 3d10 4s2 4p4 |
| 52 | Te | Tellurium | [Kr] 4d10 5s2 5p4 |
| 84 | Po | Polonium | [Xe] 4f14 5d10 6s2 6p4 |
| 116 | Lv | Livermorium | [Rn] 5f14 6d10 7s2 7p4 |
Electronic Configuration of Halogens
Fluorine, chlorine, bromine, iodine, and astatine in the periodic table belong to group 17 or the halogens family. The general electron configuration of group 17 elements is ns2 np5. Here, n = 2 to 6.
| Atomic number | Symbol | Name | Electronic configuration |
| 9 | F | Fluorine | 1s2 2s2 2p5 |
| 17 | Cl | Chlorine | [Ne] 3s2 3p5 |
| 35 | Br | Bromine | [Ar] 3d10 4s2 4p5 |
| 53 | I | Iodine | [Kr] 4d10 5s2 5p5 |
| 85 | As | Astatine | [Xe] 4f14 5d10 6s2 6p5 |
| 117 | Ts | Tennessine | [Rn] 5f14 6d10 7s2 7p5 |
Electronic Configuration of Noble Gases
Neon, argon, krypton, xenon, and radon in the periodic table belong to group 18 or noble gases. The general electron configuration of noble gases or group 18 elements is ns2 np6. Here, n = 1 to 6.
| Atomic Number | Symbol | Name | Electronic configuration |
| 2 | He | Helium | 1s2 |
| 10 | Ne | Neon | [He] 2s2 2p6 |
| 18 | Ar | Argon | [Ne] 3s2 3p6 |
| 36 | Kr | Krypton | [Ar] 3d10 4s2 4p6 |
| 54 | Xe | Xenon | [Kr] 4d10 5s2 5p6 |
| 86 | Rn | Radon | [Xe] 4f14 5d10 6s2 6p6 |
| 118 | Os | Oganesson | [Rn] 5f14 6d10 7s2 7p6 |
Electronic Configuration of d Block Elements
The elements in which the electron enters (n−1)d orbital are called d block elements. These are placed in the middle of the periodic table, between s and p block elements due to their chemical behavior like boiling point, melting by specific heat, density, ionization energy, bonding, etc.
The general electronic configuration of valence electrons of 3d, 4d, 5d, and 6d elements = ns0,1,2 (n−1)d1→10.
These elements are called transition metals or elements. The names, symbols, and valence shell electronic configuration of all the d block elements or transition metals are:
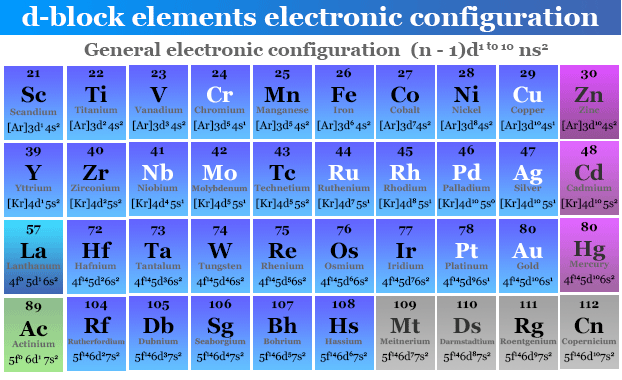
3d Block Elements Electronic Configuration
The first crystalline solid metal in the 3d series or first transition series starts with scandium and ends with zinc. Hence, when the twenty-first electron goes to the next available higher energy 3d orbital, the five 3d subshells fill with ten electrons.
The general electronic configuration of valence electrons of 3d series or first transition series like scandium, titanium, vanadium, chromium, manganese, iron, cobalt, nickel, copper, and zinc = [Ar] 4S1→2 3d1→10.
| Atomic number | Symbol | Name | Electronic configuration |
| 21 | Sc | Scandium | [Ar] 4s2 3d1 |
| 22 | Ti | Titanium | [Ar] 4s2 3d2 |
| 23 | V | Vanadium | [Ar] 4s2 3d3 |
| 24 | Cr | Chromium | [Ar] 4s1 3d5 |
| 25 | Mn | Manganese | [Ar] 4s2 3d5 |
| 26 | Fe | Iron | [Ar] 4s2 3d6 |
| 27 | Co | Cobalt | [Ar] 4s2 3d7 |
| 28 | Ni | Nickel | [Ar] 4s2 3d8 |
| 29 | Cu | Copper | [Ar] 4s1 3d10 |
| 30 | Zn | Zinc | [Ar] 4s1 3d10 |
4d Block Elements
| Atomic number | Symbol | Name | Electronic configuration |
| 39 | Y | Yttrium | [Kr] 4d1 5s2 |
| 40 | Zr | Zirconium | [Kr] 4d2 5s2 |
| 41 | Nb | Niobium | [Kr] 4d3 5s2 |
| 42 | Mo | Molybdenum | [Kr] 4d5 5s1 |
| 43 | Tc | Technetium | [Kr] 4d5 5s2 |
| 44 | Ru | Ruthenium | [Kr] 4d7 5s1 |
| 45 | Rh | Rhodium | [Kr] 4d8 5s1 |
| 46 | Pd | Palladium | [Kr] 4d10 5s0 |
| 47 | Ag | Silver | [Kr] 4d10 5s1 |
| 48 | Cd | Cadmium | [Kr] 4d10 5s2 |
5d Block Elements
| Atomic number | Symbol | Name | Electronic configuration |
| 57 | La | Lanthanum | [Xe] 5d1 6s2 |
| 72 | Hf | Hafnium | [Xe] 4f14 5d2 6s2 |
| 73 | Ta | Tantalum | [Xe] 4f14 5d3 6s2 |
| 74 | W | Tungsten | [Xe] 4f14 5d4 6s2 |
| 75 | Re | Rhenium | [Xe] 4f14 5d5 6s2 |
| 76 | Os | Osmium | [Xe] 4f14 5d6 6s2 |
| 77 | Ir | Iridium | [Xe] 4f14 5d7 6s2 |
| 78 | Pt | Platinum | [Xe] 4f14 5d9 6s1 |
| 79 | Au | Gold | [Xe] 4f14 5d10 6s1 |
| 80 | Hg | Mercury | [Xe] 4f14 5d10 6s2 |
6d Block Elements
| Atomic number | Symbol | Name | Electronic configuration |
| 89 | Ac | Actinium | [Rn] 6d1 7s2 |
| 104 | Rf | Rutherfordium | [Rn] 5f14 6d2 7s2 |
| 105 | Db | Dubnium | [Rn] 5f14 6d3 7s2 |
| 106 | Sg | Seaborgium | [Rn] 5f14 6d4 7s2 |
| 107 | Bh | Bohrium | [Rn] 5f14 6d5 7s2 |
| 108 | Hs | Hassium | [Rn] 5f14 6d6 7s2 |
| 109 | Mt | Meitnerium | [Rn] 5f14 6d7 7s2 |
| 110 | Ds | Darmstadtium | [Rn] 5f14 6d9 7s1 |
| 111 | Rg | Roentgenium | [Rn] 5f14 6d10 7s1 |
| 112 | Cn | Copernicium | [Rn] 5f14 6d10 7s2 |
Electron Configuration Exceptions
Chromium, and copper, the 3d elements of our environment reveal their general form of electron configuration trends in the periodic table. The general electronic configuration of chromium and copper is [Ar]4s2 3d4 and [Ar]4s2 3d9.
In the periodic table elements, the half-filled and filled orbital’s electron configuration formula is relatively more stable than the partially filled orbitals. Therefore, the 3d orbital of chromium and copper rearranges to form new electronic configurations to gain extra chemical stability by exchange energy. These electronic configurations are represented as [Ar]4s1 3d5 and [Ar] 4s1 3d10.
Electron Configuration of f Block Elements
4f Block Elements
| Atomic number | Symbol | Name | Electronic configuration |
| 58 | Ce | Cerium | [Xe] 4f15d16s2 |
| 59 | Pr | Praseodymium | [Xe] 4f36s2 |
| 60 | Nd | Neodymium | [Xe] 4f46s2 |
| 61 | Pm | Promethium | [Xe] 4f56s2 |
| 62 | Sm | Samarium | [Xe]4f66s2 |
| 63 | Eu | Europium | [Xe] 4f76s2 |
| 64 | Gd | Gadolinium | [Xe] 4f75d16s2 |
| 65 | Tb | Terbium | [Xe] 4f96s2 |
| 66 | Dy | Dysprosium | [Xe] 4f106s2 |
| 67 | Ho | Holmium | [Xe] 4f116s2 |
| 68 | Er | Erbium | [Xe] 4f126s2 |
| 69 | Tm | Thulium | [Xe]4f136s2 |
| 70 | Yb | Ytterbium | [Xe] 4f146s2 |
| 71 | Lu | Lutetium | [Xe] 4f145d16s2 |
5f Block Elements
| Atomic number | Symbol | Name | Electronic configuration |
| 90 | Th | Thorium | [Rn] 6d27s2 |
| 91 | Pa | Protactinium | [Rn] 5f26d17s2 |
| 92 | U | Uranium | [Rn] 5f36d17s2 |
| 93 | Np | Neptunium | [Rn] 5f46d17s2 |
| 94 | Pu | Plutonium | [Rn] 5f67s2 |
| 95 | Am | Americium | [Rn] 5f77s2 |
| 96 | Cm | Curium | [Rn] 5f76d17s2 |
| 97 | Bk | Berkelium | [Rn] 5f97s2 |
| 98 | Cf | Californium | [Rn] 5f107s2 |
| 99 | Es | Einsteinium | [Rn] 5f117s2 |
| 100 | Fm | Fermium | [Rn] 5f127s2 |
| 101 | Md | Mendelevium | [Rn] 5f137s2 |
| 102 | No | Nobelium | [Rn] 5f147s2 |
| 103 | Lw | Lawrencium | [Rn] 5f147s27p1 |