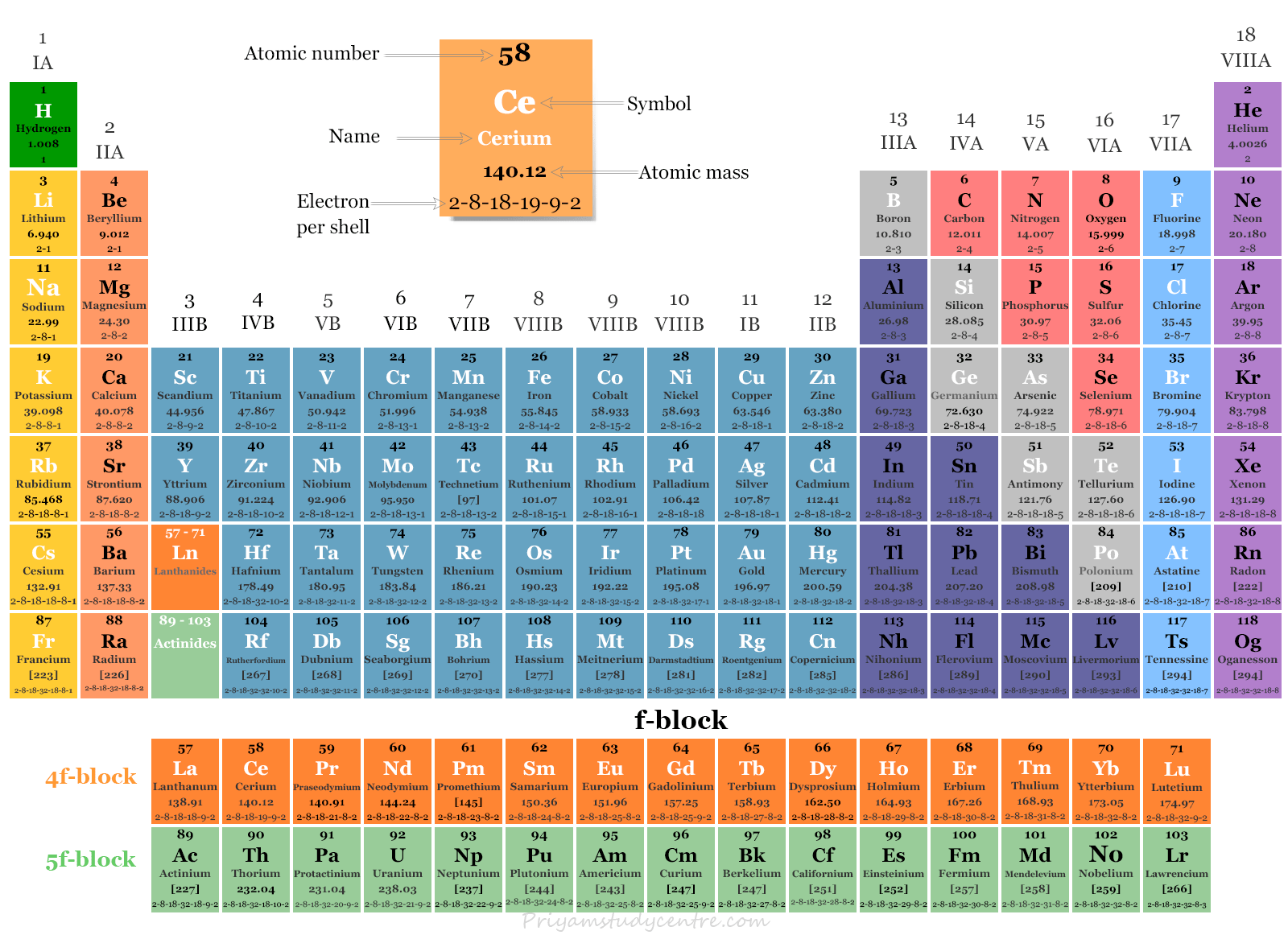
f Block Elements on Periodic Table
f block elements appear in two chemical series like 4f block names as lanthanides or rare earth elements and 5f block names as actinides or actinoids in the periodic table. The 4f-block chemical elements or lanthanides constitute the first inner transition series while actinides constitute the second inner transition metals series in chemistry. The electronic configuration of the f-block chemical elements (lanthanum and actinium) has been done by filling electrons in deep-seated 4f and 5f orbital with increasing atomic number.
The general valence shell electronic configuration of f block elements (lanthanum and actinium series) is (n − 2)f0, 2 to 14 (n − 1)d0 to 2 ns2. The trivalent oxidation number or state of all f-block elements is a stable or common oxidation state.
Why are f Block Elements Called Inner Transition Elements?
f-block is also called inner transition metals or elements because the electron is added to the deep-seated f-orbital with the increasing atomic number of lanthanides and actinides. Therefore, the outer orbital contains 6s or 7s-electrons and the inner energy orbital contains f-electrons for lanthanides and actinides.
f Block Elements Names and Symbols
The f-block elements are classified into 4f and 5f series of elements corresponding to filling 4f and 5f orbitals of (n − 2) main shell. The names and symbols of all f-block elements are given below the two pictures.
What are Lanthanides?
The 4f block contains fourteen chemical elements cerium to lutetium with an atomic number from 58 to 71. These f-block chemical elements are known as Lanthanum or rare-earth elements because they appear after lanthanum.
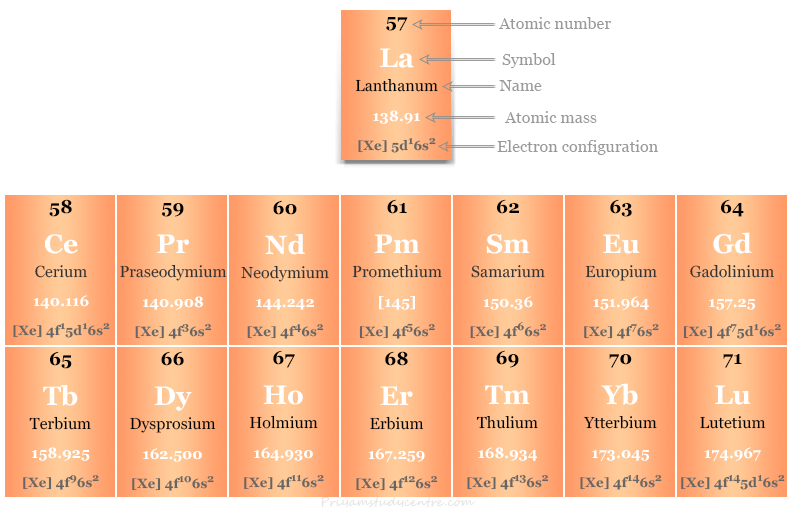
The element lanthanum is also placed with the lanthanide series because the chemical and physical properties of fifteen elements, 57La to 71Lu are very similar.
What are Actinides?
The 5f block contains fourteen chemical elements thorium to lawrencium with atomic numbers 90 to 103. These f-block elements are known as actinides because they appear after actinium.
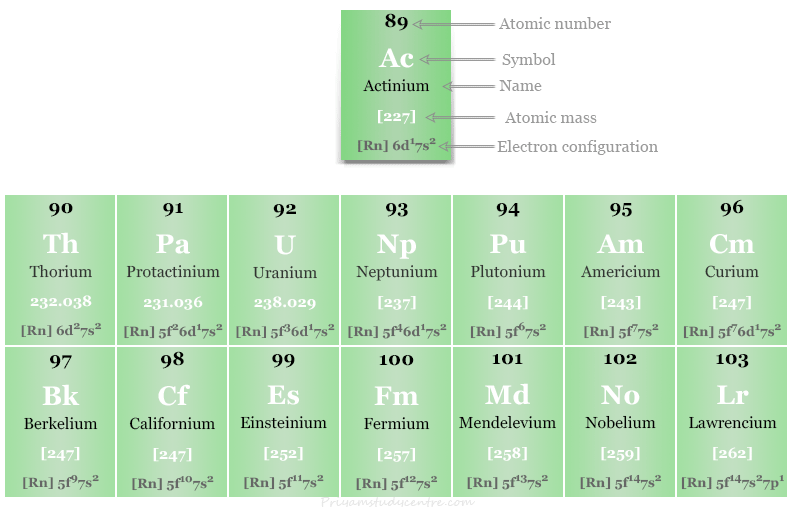
Actinium is also placed with the actinide series because the physical and chemical properties of fifteen elements, 89Ac to 103Lw are very similar.
Properties of f Block Elements
The f-block chemical elements like lanthanides behave as active metals. Therefore, the redox reaction potential of these elements is comparable to alkaline earth metals. All the f-block metals act as strong reducing agents and chemical reactions with acids by the liberation of hydrogen ions. Hence they absorb hydrogen ions from low pH scale solutions to form interstitial hydrides.
Although important similarities exist between the two series of f-block elements but very important differences also occur. These differences occur due to lower bond energies, ionization energy, and less effective shielding electron in 5f block elements than the 4f-block elements.
The standard electrode potentials for f-block lanthanides become more positive with the increasing atomic number. But for actinium, electrode potential values become more positive from actinium to uranium and become less positive till Americium.
Electronic Configuration of f Block Elements
The general valence shell electronic configuration of f block elements (lanthanum and actinium series) is
(n − 2)f0, 2 to 14 (n − 1)d0 to 2 ns2
For example, the valence shell electronic configuration of 4f block elements promethium (atomic number 61) is 4f5 5d0 6s2.
4f Block (Lanthanides) Elements in Periodic Table
The 4f block elements in the periodic table have been variously called rare earth, lanthanum’s, and lanthanum.
The name rare-earth for f-block has been given because they are originally extracted from oxides for which the ancient name earth and which are considered to be rare in earth’s environment. The term rare earth is now avoided because many of these elements are no longer rare but are abundant.
Electronic Configuration of Lanthanides
The general valence shell electronic configuration of lanthanides
[Xe] 4f0, 2 to 14 5d0 to1 6s2
Therefore, the valence shell electronic configuration of praseodymium is [Xe] 4f3 5d0 6s2
| Atomic number | Symbol | Name | Electronic configuration |
| 58 | Ce | Cerium | [Xe] 4f1 5d1 6s2 |
| 59 | Pr | Praseodymium | [Xe] 4f3 6s2 |
| 60 | Nd | Neodymium | [Xe] 4f4 6s2 |
| 61 | Pm | Promethium | [Xe] 4f5 6s2 |
| 62 | Sm | Samarium | [Xe] 4f6 6s2 |
| 63 | Eu | Europium | [Xe] 4f7 6s2 |
| 64 | Gd | Gadolinium | [Xe] 4f7 5d1 6s2 |
| 65 | Tb | Terbium | [Xe] 4f9 6s2 |
| 66 | Dy | Dysprosium | [Xe] 4f10 6s2 |
| 67 | Ho | Holmium | [Xe] 4f11 6s2 |
| 68 | Er | Erbium | [Xe] 4f12 6s2 |
| 69 | Tm | Thulium | [Xe] 4f13 6s2 |
| 70 | Yb | Ytterbium | [Xe] 4f14 6s2 |
| 71 | Lu | Lutetium | [Xe] 4f14 5d16s2 |
Cerium, Gadolinium, and Lutetium
f-block chemical elements like cerium, gadolinium, and Lutetium in the periodic table contain one electron in a 5d orbital with atomic numbers 58, 64, and 87. Therefore, the electron configuration of cerium, gadolinium, and lutetium is outside of the general electronic configuration. Because electrons of similar spin developed an exchange interaction which leads to stabilizing the system.
The f-electrons of similar spin, repulsion is less by an amount called the exchange energy of an electron. But the greater the number of f-electrons with parallel spins the greater of exchanged interaction and greater stability. This is the basis of Hound’s rules and is used to derive the electronic arrangement of Cerium, Gadolinium, and Lutetium.
Electronic Configuration of Cerium
The electron configuration of cerium,
[Xe] 4f1 5d1 6s2
The cerium atom contains one electron in a 5d orbital. But 4f and 5d energy levels are very close in terms of energy. Therefore, the half-filled orbital f-block chemical element like cerium is slightly more stable than the orbital with one additional electron.
Electronic Configuration of Gadolinium
The electron configuration of f-block chemical elements like gadolinium is,
[Xe] 4f7 5d1 6s2
Gadolinium contains seven electrons with a parallel spin in the seven f-orbitals with the maximum stability of the f orbital. Here the half-filled energy level stabilizes by exchange energy in the 4f orbital of the gadolinium atom.
Electronic Configuration of Lutetium
Lutetium also has a valence orbital electronic configuration, f14 d1, where the last electrons have added the capacity of the f-shell. Therefore, the electronic configuration f-block chemical elements like Lutetium, [Xe] 4f14 5d1 6s2.
Electronic Configuration of Praseodymium
Praseodymium in f-block possesses electronic configuration 4f3 6s2 instead of the expected one 4f2 5d1 6s2. This can be explained by (n + l) rules. The orbital which has a higher value of (n + l) is the higher energy orbital.
- For 4f orbital, (n + l) = 4 + 3 = 7
- For 5d orbital, (n + l) = 5 + 7 = 7
For 4f and 5d-orbital, the sum of the principal and the azimuthal quantum number is the same. In this case, the highest number of principal quantum numbers is the higher energy quantum systems of an atom. Therefore, 5d-orbital is a higher energy quantum shell.
Again electrons are fed into orbitals in order of increasing energy until all the electrons have been accommodated. Hence the electron filling process for f block element praseodymium f-electron filling first and possesses electronic configuration 4f3 6s2.
Common Oxidation State of Lanthanides
The trivalent oxidation state is the common oxidation state of elements like lanthanum. Because the removal of three electrons from lanthanides is easier than the removal of greater than three electrons. The ground state electronic configuration of the lanthanum atom and trivalent lanthanum ion is [Xe] 4f0 5d1 6s2 and [Xe] 4f0 respectively.
The stability of electrons in the f orbital of lanthanides is greater than the d and s electrons. Therefore, the f electrons can not participate in the chemical bonding and the +3 oxidation number or state is common for lanthanides.
5f Block Elements or Actinides
The second series of 5f block chemical elements results from the filling of 5f-orbital. Actinides in the f block consist of chemical elements like thorium to lawrencium with atomic numbers 90 to 103.
General Electronic Configuration of Actinides
The general electronic configuration of 5f-block elements or actinides is
[Rn] 5f0, 2 to 14 6d0,1,2 7s2
Therefore, the electronic configuration of uranium is [Rn] 5f3 6d1 7s2.
| Atomic number | Symbol | Name | Electronic configuration |
| 90 | Th | Thorium | [Rn] 6d2 7s2 |
| 91 | Pa | Protactinium | [Rn] 5f2 6d1 7s2 |
| 92 | U | Uranium | [Rn] 5f3 6d1 7s2 |
| 93 | Np | Neptunium | [Rn] 5f4 6d1 7s2 |
| 94 | Pu | Plutonium | [Rn] 5f6 7s2 |
| 95 | Am | Americium | [Rn] 5f7 7s2 |
| 96 | Cm | Curium | [Rn] 5f7 6d1 7s2 |
| 97 | Bk | Berkelium | [Rn] 5f9 7s2 |
| 98 | Cf | Californium | [Rn] 5f10 7s2 |
| 99 | Es | Einsteinium | [Rn] 5f117s2 |
| 100 | Fm | Fermium | [Rn] 5f12 7s2 |
| 101 | Md | Mendelevium | [Rn] 5f13 7s2 |
| 102 | No | Nobelium | [Rn] 5f14 7s2 |
| 103 | Lr | Lawrencium | [Rn] 5f14 7s2 7p1 |
Position of 5f Block Elements (Actinides) in Periodic Table
The highest atomic number of naturally occurring actinides (f-block elements) = 92. For this reason, uranium occupies the last position in the periodic table for a very long time.
Since 1940 seven chemical elements from neptunium to lawrencium have been identified and synthesized by the transformation of naturally occurring elements by the emission of alpha, beta, or gamma rays.
These men made eleven radioactive isotopes that are placed beyond uranium in the periodic table and are collectively called trans-uranium chemical elements. The absorption spectra of f-block actinides ion and crystalline solid chemical elements contain narrow bands with visible, near-ultraviolet, and near-infrared spectra in electromagnetic spectrum radiation.