Electron Definition in Chemistry
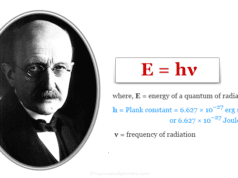
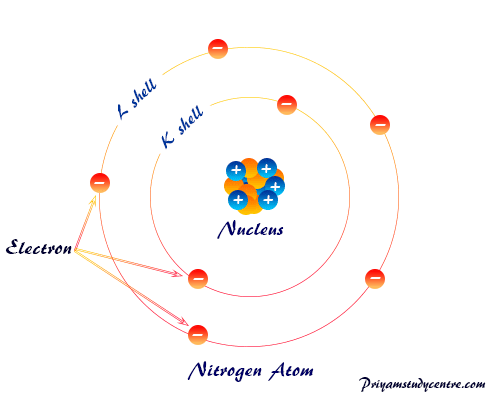
Electron in chemistry, the lightest subatomic or elementary particle of an atom carries a negative charge, −1.602176634 × 10−19 coulomb or −4.8 × 10−10 esu. Like other particles, electrons have mass, energy, momentum (particle properties), and wavelength or frequency (wave properties) derived from the de-Broglie relation (λ = h/mν = h/P). Electrons are discovered by an experiment during the conduction of electricity through gases at low pressure. The periodic table is drawn up by chemists primarily based on the atomic number and electron distribution or arrangement. The distribution of electrons in different shells not only provides the size of an atom but it also provides properties like chemical bonding, ionization energy, electron affinity, electronegativity, oxidation number or state, electromagnetic spectrum, thermal conductivity, etc. The arrangement or distribution of electrons in a nitrogen atom is given below the picture.
 The rest mass of an electron is 9.1093837015 × 10−28 g or 9.1093837015 × 10−31 kg. However, it is approximately 1/1836 of the mass of the proton particle.
The rest mass of an electron is 9.1093837015 × 10−28 g or 9.1093837015 × 10−31 kg. However, it is approximately 1/1836 of the mass of the proton particle.
Who Discovered Electrons?
The electrons were discovered by the British physicist J.J. Thomson in 1897 during the investigation of the properties of the cathode rays over twenty years. Gas discharge tubes contain a positive electrode (anode) and a negative electrode (cathode) with provisions of evacuation given below the picture,
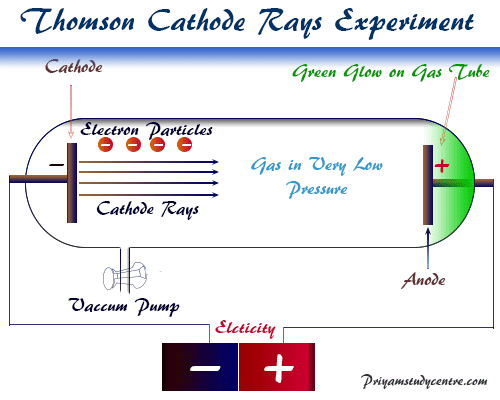
Under normal conditions, gases are poor conductors of electricity but at low pressure, conduction of gases occurs. When the gas discharge tubes are partially evacuated and the electrodes are connected to the source of high-voltage electricity, an electric current flows through the tubes.
The flow of current produces glow or rays of light that originate from the cathode surface and travel from cathode to anode. These rays are called cathode rays.
Thomson showed that the rays or electromagnetic radiation can be bent from their path both by the electric and magnetic fields. From the above facts, he proved that the cathode rays are made up of negatively charged subatomic particles or electrons of an atom.
Thomson measured the charge to mass or e/m ratio (1.76 × 10−8) of an electron. However, the absolute charge was determined by the American physicists Robert Millikan and Harvey Fletcher in their oil-drop experiment.
Charge of Electron
Electrons are negatively charged elementary subatomic particles of an atom. Hence the negative charge carries by an electron,
e = − 4.8 × 10−10 esu
= − 1.602176634 × 10−19 coulomb (C)
Mass of Electron
Mass of an electron = m and charge = e. Charge by mass (e/m) ratio for electron = 1.76 × 108 coulomb/g.
From the above formula, the mass of an electron particle,
me = (1.602176634 × 10−19)/(1.76 × 108) g
= 9.1093837015 × 10−28 g
= 9.1093837015 × 10−31 kg
How charge of electron is determined?
Electrolysis of silver from an aqueous solution of silver salt is a suitable process for the determination of the charge of electrons. A constant current I (ampere) passes through the silver nitrate solution during the time t.
The amount of electricity required to decompose one gram equivalent substances is called one faraday.
∴ 1 F = 96496 ≈ 96500 coulombs
Therefore, the charge of 96500 coulombs can be considered as the charge carried by a mole of electrons.
Therefore, the charge associated with an electron,
= (96500)/(6.02 × 1023)
= 1.60 × 10−19 coulomb = 4.8 × 10-10 esu
Electron Arrangement
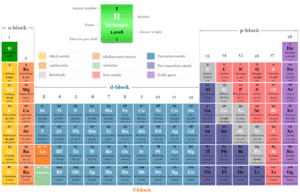
The periodic table is drawn up by chemists generally based on the atomic number and electron distribution or arrangement in chemistry. The atomic number represents the net positive charge on the nucleus or the number of electrons surrounding the nucleus of an atom in its normal neutral condition.
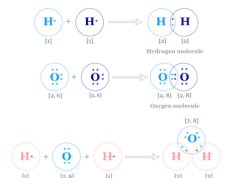
The Rutherford model and Bohr model provide the basic electronic structure of an atom. However, the modern structure of atoms is based on the electron arrangement of periodic table elements in different orbitals. According to modern atomic structure, the distribution of atomic orbitals where electrons are arranged is given below the picture,

Electron Arrangement in Orbitals
| Electron arrangement in orbitals | ||
| Element | Electron Per Shell | |
| Atomic number | Name and Symbol | (K, L, M, N, O, P, Q) |
| 1 | Hydrogen (H) | 1 |
| 2 | Helium (He) | 2 |
| 3 | Lithium (Li) | 2, 1 |
| 4 | Beryllium (Be) | 2, 2 |
| 5 | Boron (B) | 2, 3 |
| 6 | Carbon (C) | 2, 4 |
| 7 | Nitrogen (N) | 2, 5 |
| 8 | Oxygen (O) | 2, 6 |
| 9 | Fluorine (F) | 2, 7 |
| 10 | Neon (Ne) | 2, 8 |
| 11 | Sodium (Na) | 2, 8, 1 |
| 12 | Magnesium (Mg) | 2, 8, 2 |
| 13 | Aluminum (Al) | 2, 8, 3 |
| 14 | Silicon (Si) | 2, 8, 4 |
| 15 | Phosphorus (P) | 2, 8, 5 |
| 16 | Sulfur (S) | 2, 8, 6 |
| 17 | Chlorine (Cl) | 2, 8, 7 |
| 18 | Argon (Ar) | 2, 8, 8 |
| 19 | Potassium (K) | 2, 8, 8, 1 |
| 20 | Calcium (Ca) | 2, 8, 8, 2 |
| 21 | Scandium (Sc) | 2, 8, 9, 2 |
| 22 | Titanium (Ti) | 2, 8, 10, 2 |
| 23 | Vanadium (V) | 2, 8, 11, 2 |
| 24 | Chromium (Cr) | 2, 8, 13, 1 |
| 25 | Manganese (Mn) | 2, 8, 13, 2 |
| 26 | Iron (Fe) | 2, 8, 14, 2 |
| 27 | Cobalt (Co) | 2, 8, 15, 2 |
| 28 | Nickel (Ni) | 2, 8, 16, 2 |
| 29 | Copper (Cu) | 2, 8, 18, 1 |
| 30 | Zinc (Zn) | 2, 8, 18, 2 |
| 31 | Gallium (Ga) | 2, 8, 18, 3 |
| 32 | Germanium (Ge) | 2, 8, 18, 4 |
| 33 | Arsenic (Ar) | 2, 8, 18, 5 |
| 34 | Selenium (Se) | 2, 8, 18, 6 |
| 35 | Bromine (Br) | 2, 8, 18, 7 |
| 36 | Krypton (Kr) | 2, 8, 18, 8 |
| 37 | Rubidium (Ru) | 2, 8, 18, 8, 1 |
| 38 | Strontium (Sr) | 2, 8, 18, 8, 2 |
| 39 | Yttrium (Y) | 2, 8, 18, 9, 2 |
| 40 | Zirconium (Zr) | 2, 8, 18, 10, 2 |
| 41 | Niobium (Nb) | 2, 8, 18, 12, 1 |
| 42 | Molybdenum (Mo) | 2, 8, 18, 13, 1 |
| 43 | Technetium (Tc) | 2, 8, 18, 13, 2 |
| 44 | Ruthenium (Ru) | 2, 8, 18, 15, 1 |
| 45 | Rhodium (Rh) | 2, 8, 18, 16, 1 |
| 46 | Palladium (Pd) | 2, 8, 18, 18 |
| 47 | Silver (Ag) | 2, 8, 18, 18, 1 |
| 48 | Cadmium (Cd) | 2, 8, 18, 18, 2 |
| 49 | Indium (In) | 2, 8, 18, 18, 3 |
| 50 | Tin (Sn) | 2, 8, 18, 18, 4 |
| 51 | Antimony (Sb) | 2, 8, 18, 18, 5 |
| 52 | Tellurium (Te) | 2, 8, 18, 18, 6 |
| 53 | Iodine (I) | 2, 8, 18, 18, 7 |
| 54 | Xenon (Xe) | 2, 8, 18, 18, 8 |
| 55 | Caesium (Cs) | 2, 8, 18, 18, 8, 1 |
| 56 | Barium (Ba) | 2, 8, 18, 18, 8, 2 |
| 57 | Lanthanum (La) | 2, 8, 18, 18, 9, 2 |
| 58 | Cerium (Ce) | 2, 8, 18, 19, 9, 2 |
| 59 | Praseodymium (pr) | 2, 8, 18, 21, 8, 2 |
| 60 | Neodymium (Nd) | 2, 8, 18, 22, 8, 2 |
| 61 | Promethium (Pm) | 2, 8, 18, 23, 8, 2 |
| 62 | Samarium (Sm) | 2, 8, 18, 24, 8, 2 |
| 63 | Europium (Eu) | 2, 8, 18, 25, 8, 2 |
| 64 | Gadolinium (Gd) | 2, 8, 18, 25, 9, 2 |
| 65 | Terbium (Tb) | 2, 8, 18, 27, 8, 2 |
| 66 | Dysprosium (Dy) | 2, 8, 18, 28, 8, 2 |
| 67 | Holmium (Ho) | 2, 8, 18, 29, 8, 2 |
| 68 | Erbium (Er) | 2, 8, 18, 30, 8, 2 |
| 69 | Thulium (Tm) | 2, 8, 18, 31, 8, 2 |
| 70 | Ytterbium (Yb) | 2, 8, 18, 32, 8, 2 |
| 71 | Lutetium (Lu) | 2, 8, 18, 32, 9, 2 |
| 72 | Hafnium (Hf) | 2, 8, 18, 32, 10, 2 |
| 73 | Tantalum (Ta) | 2, 8, 18, 32, 11, 2 |
| 74 | Tungsten (W) | 2, 8, 18, 32, 12, 2 |
| 75 | Rhenium (Re) | 2, 8, 18, 32, 13, 2 |
| 76 | Osmium (Os) | 2, 8, 18, 32, 14, 2 |
| 77 | Iridium (Ir) | 2, 8, 18, 32, 15, 2 |
| 78 | Platinum (Pt) | 2, 8, 18, 32, 17, 1 |
| 79 | Gold (Au) | 2, 8, 18, 32, 18, 1 |
| 80 | Mercury (Hg) | 2, 8, 18, 32, 18, 2 |
| 81 | Thallium (Tl) | 2, 8, 18, 32, 18, 3 |
| 82 | Lead (Pb) | 2, 8, 18, 32, 18, 4 |
| 83 | Bismuth (Bi) | 2, 8, 18, 32, 18, 5 |
| 84 | Polonium (Po) | 2, 8, 18, 32, 18, 6 |
| 85 | Astatine (At) | 2, 8, 18, 32, 18, 7 |
| 86 | Radon (Rn) | 2, 8, 18, 32, 18, 8 |
| 87 | Francium (Fr) | 2, 8, 18, 32, 18, 8, 1 |
| 88 | Radium (Ra) | 2, 8, 18, 32, 18, 8, 2 |
| 89 | Actinium (Ac) | 2, 8, 18, 32, 18, 9, 2 |
| 90 | Thorium (Th) | 2, 8, 18, 32, 18, 10, 2 |
| 91 | Protactinium (Pa) | 2, 8, 18, 32, 20, 9, 2 |
| 92 | Uranium (U) | 2, 8, 18, 32, 21, 9, 2 |
| 93 | Neptunium (Np) | 2, 8, 18, 32, 22, 9, 2 |
| 94 | Plutonium (Pu) | 2, 8, 18, 32, 24, 8, 2 |
| 95 | Americium (Am) | 2, 8, 18, 32, 25, 8, 2 |
| 96 | Curium (Cm) | 2, 8, 18, 32, 25, 9, 2 |
| 97 | Berkelium (Bk) | 2, 8, 18, 32, 27, 8, 2 |
| 98 | Californium (Cf) | 2, 8, 18, 32, 28, 8, 2 |
| 99 | Einsteinium (Es) | 2, 8, 18, 32, 29, 8, 2 |
| 100 | Fermium (Fm) | 2, 8, 18, 32, 30, 8, 2 |
| 101 | Mendelevium (Md) | 2, 8, 18, 32, 31, 8, 2 |
| 102 | Nobelium (No) | 2, 8, 18, 32, 32, 8, 2 |
| 103 | Lawrencium (Lr) | 2, 8, 18, 32, 32, 8, 3 |
| 104 | Rutherfordium (Rf) | 2, 8, 18, 32, 32, 10, 2 |
| 105 | Dubnium (Db) | 2, 8, 18, 32, 32, 11, 2 |
| 106 | Seaborgium (Sg) | 2, 8, 18, 32, 32, 12, 2 |
| 107 | Bohrium (Bh) | 2, 8, 18, 32, 32, 13, 2 |
| 108 | Hassium (Hs) | 2, 8, 18, 32, 32, 14, 2 |
| 109 | Meitnerium (Mt) | 2, 8, 18, 32, 32, 15, 2 |
| 110 | Darmstadtium (Ds) | 2, 8, 18, 32, 32, 16, 2 |
| 111 | Roentgenium (Rg) | 2, 8, 18, 32, 32, 17, 2 |
| 112 | Copernicium (Cn) | 2, 8, 18, 32, 32, 18, 2 |
| 113 | Nihonium (Nh) | 2, 8, 18, 32, 32, 18, 3 |
| 114 | Flerovium (Fl) | 2, 8, 18, 32, 32, 18, 4 |
| 115 | Moscovium (Mc) | 2, 8, 18, 32, 32, 18, 5 |
| 116 | Livermorium (Lv) | 2, 8, 18, 32, 32, 18, 6 |
| 117 | Tennessine (Ts) | 2, 8, 18, 32, 32, 18, 7 |
| 118 | Oganesson (Og) | 2, 8, 18, 32, 32, 18, 8 |
Electrons Arrangement in Atoms
The electron arrangement in the orbitals of atoms in a definite order provides the electronic configuration of periodic table elements.
The noble gases (helium, neon, krypton, and xenon) are chemically inert because they contain stable electron arrangements in their atoms. From helium to xenon chemical reactivity increases because the size of the atom increases and ionization energy decreases.
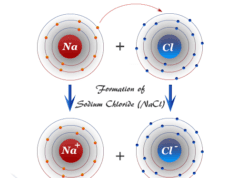
Group 17 elements like fluorine, chlorine, and bromine are highly electronegative and reactive non-metals because they have only one electron short of the next noble gas. Therefore, they can easily gain one electron to form the next noble gas configuration.
The electrons arrangement in the atoms of alkali metals (lithium, sodium, and potassium) shows that they are highly electropositive or reactive because they can easily lose one electron from the valence shell.