Transition Metals on the Periodic Table
Transition metals or transition elements on the periodic table are the d-block chemical elements lying between p-and s-block elements. These elements either in their atomic state or in any of their common oxidation number or state have partially filled (n-1)d orbital. The valence shell electronic configuration of d-block elements or transition metals, (n−1)d1 to 10 ns0, 1, 2. According to the definition of transition metal, copper, silver, and gold should be excluded from d-block elements on the periodic table. Since these elements do not have partially filled d-orbitals in their atomic or common oxidation state. The f-block elements (lanthanides or rare-earth elements and actinides) in the periodic table are also called inner transition metals. The f block elements are named inner transition metals because the additional electron enters into (n−2)f orbitals.
The metals zinc, cadmium, mercury, and palladium also do not contain partially filled d-orbital in their atomic or common +2 oxidation state. Due to similar properties and to maintain a rational classification, these elements are generally studied with d-block or transition metals.
Electronic Configuration of Transition Elements
All the transition elements or d-block elements are classified into four series such as 3d, 4d, 5d, and 6d. Each 3d, 4d, 5d, and 6d series has ten chemical elements.
The general valence shell electronic configuration of transition elements,
(n-1)d1 to 10 ns0, 1, 2
First Transition Series
| Transition element | Symbol | Atomic number | Electronic configuration |
| Scandium | Sc | 21 | [Ar] 3d1 4s2 |
| Titanium | Ti | 22 | [Ar] 3d2 4s2 |
| Vanadium | V | 23 | [Ar] 3d3 4s2 |
| Chromium | Cr | 24 | [Ar] 3d5 4s1 |
| Manganese | Mn | 25 | [Ar] 3d5 4s2 |
| Iron | Fe | 26 | [Ar] 3d6 4s2 |
| Cobalt | Co | 27 | [Ar] 3d7 4s2 |
| Nickel | Ni | 28 | [Ar] 3d8 4s2 |
| Copper | Cu | 29 | [Ar] 3d10 4s1 |
| Zinc | Zn | 30 | [Ar] 3d10 4s2 |
Second Transition Series
| Transition element | Symbol | Atomic number | Electronic configuration |
| Yttrium | Y | 39 | [Kr] 4d1 5s2 |
| Zerconium | Zr | 40 | [Kr] 4d2 5s2 |
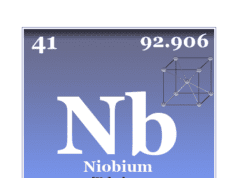
| Niobium | Nb | 41 | [Kr] 4d4 5s1 |
| Molybdenum | Mo | 42 | [Kr] 4d5 5s1 |
| Technetium | Tc | 43 | [Kr] 4d5 5s2 |
| Ruthenium | Ru | 44 | [Kr] 3d7 5s1 |
| Rhodium | Rh | 45 | [Kr] 3d7 4s2 |
| Palladium | Pd | 46 | [Kr] 4d10 5s0 |
| Silver | Ag | 47 | [Kr] 4d10 5s1 |
| Cadmium | Cd | 48 | [Kr] 4d10 5s2 |
Third Transition Series
| Transition element | Symbol | Atomic number | Electronic configuration |
| Lanthanum | La | 57 | [Xe] 4f0 5d1 6s2 |
| Hafnium | Hf | 72 | [Xe] 4f14 5d2 6s2 |
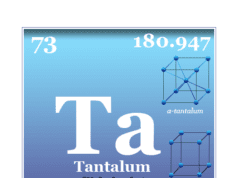
| Tantalum | Ta | 73 | [Xe] 4f14 5d3 6s2 |
| Tungsten | W | 74 | [Xe] 4f14 5d4 6s2 |
| Rhenium | Re | 75 | [Xe] 4f14 5d5 6s2 |
| Osmium | Os | 76 | [Xe] 4f14 5d6 6s2 |
| Iridium | Ir | 77 | [Xe] 4f14 5d7 6s2 |
| Platinum | Pt | 78 | [Xe] 4f14 5d9 6s1 |
| Gold | Au | 79 | [Xe] 4f14 5d10 6s1 |
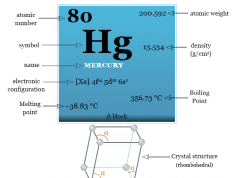
| Mercury | Hg | 80 | [Xe] 4f14 5d10 6s2 |
Fourth Transition Series
| Transition element | Symbol | Atomic number | Electronic configuration |
| Actinium | Ac | 89 | [Rn] 5f0 6d1 7s2 |
| Rutherfordium | Rf | 104 | [Rn] 5f14 6d2 7s2 |
| Dubnium | Db | 105 | [Rn] 5f14 6d3 7s2 |
| Seaborgium | Sg | 106 | [Rn] 5f14 6d4 7s2 |
| Bohrium | Bh | 107 | [Rn] 5f14 6d5 7s2 |
| Hassium | Hs | 108 | [Rn] 5f14 6d6 7s2 |
| Meitnerium | Mt | 109 | [Rn] 5f14 6d7 7s2 |
| Darmstadtium | Ds | 110 | [Rn] 5f14 6d8 7s2 |
| Roentgenium | Rg | 111 | [Rn] 5f14 6d9 7s2 |
| Copernicium | Cn | 112 | [Rn] 5f14 6d10 7s2 |
Properties of Transition Elements
The properties of transition elements of a given period are not so much different from one another. The reason for this fact lies in the electronic configuration of transition elements.
The general electronic configuration of transition metals differs one from another only in the number of electrons in the d-orbital. The number of electrons in the outermost shell is invariably 0, 1, or 2.
Metallic Character of Transition Elements
All the transition elements are metals due to their small number of electrons in the outermost quantum shell. They are hard, malleable, and ductile. They have formed all four types of crystalline solids − face-centered cubic, hexagonal closed packed, body-centered cubic, and face-centered cubic crystal lattice.
Metallic and covalent bonding both exist in atoms of transition metals. Due to the presence of incomplete d-orbital, most of the transition metals combine with other elements by covalent chemical bonding. These metals are good conductors of heat and electricity.
Transition Metals Melting Point Trends
The transition elements have very high melting and boiling points compared to s- and p-block elements.
The metals zinc, cadmium, and mercury have relatively low values due to completely filled d-orbitals. No unpaired electrons are available for covalent bonding among the atoms of zinc, cadmium, and mercury.
The other transition metal contains an incompletely filled d-orbital for covalent bonding.
Atomic and Ionic Radii of Transition Elements
The atomic and ionic radii decrease generally on moving from left to right in the period. This is due to the fact that an increase in nuclear charge tends to attract the electron clouds towards the nucleus of an atom.
The atomic radii of chromium to copper are very close to one another. Due to the simultaneous addition of shielding electron to 3d-level reverse effect on outer 4s-electrons. The ionic radii of M+2 and M+3 ions follow the same trends as atomic radii.
Ionization Energy of Transition Metals
The first ionization energy of transition metals lies between the values of s- and p-block elements. The first ionization energy values lie between 5 to 10 electron volts.
In the case of transition metals, the addition of extra shielding electron shields or decreases the inward pulls of the positive nucleus and ns electrons. The effects of the increasing nuclear charge and shielding effects oppose each other.
On account of these counter effects, the ionization potentials increase slowly on moving in a period of the first transition series.
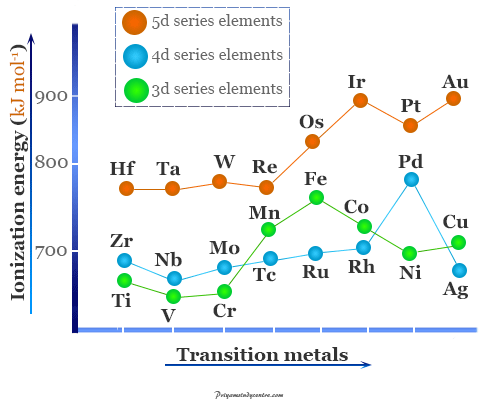
- From the above picture, it is clearly indicated that the first ionization energies of Ti, V, and Cr differ slightly from one another. Similarly, the values of Fe, Co, Ni, and Cu are fairly close to one another.
- Second ionization energy is seen to increase more or less regularly with the increasing atomic number of d-block or transition elements. The values of second ionization energy chromium and copper are higher than that of their neighbor transition metals due to the extra stability of Cu+ and Cr+ ions.
Oxidation Number of Transition Metals
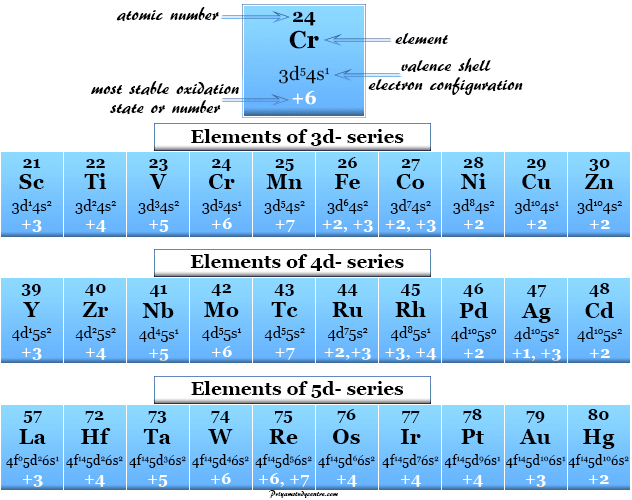
One of the most important properties that distinguish transition metals from non-transition elements is variable oxidation numbers or states.
This unique property is due to the fact that the energy levels of 3d, 4d, and 5d orbitals are close to the 4s, 5s, and 6s orbitals respectively. Therefore, in addition to ns electrons, the various numbers of (n−1)d electrons are also lost to show various oxidation states.
Catalytic Activity of Transition Metals
Most of the transition metals and their compounds are used as good chemical catalyst in different types of reactions. Some examples are
- Vanadium pentoxide (V2O5) is used in the manufacture of sulfuric acid in the contact process.
- Finely divided powdered nickel or active nickel is used in hydrogenation reactions of organic compounds.
- Spongy platinum is used in the conversion of sulfur dioxide to sulfur trioxide or the production of nitric acid by the Ostwald process.
- Transition metal, iron is used in the manufacture of ammonia by the Haber process.