Rutherford Model of an Atom
Rutherford model or nuclear planetary model of an atom in chemistry or physics describes by New Zealand-born physicist Ernest Rutherford in his gold foil experiment on thin metal. Ernest Rutherford and his students describe the gold foil experiment model of an atom to provide an atomic structure in two parts, the nucleus of an atom and extranuclear electrons.
The presence of free charge electrons observed by Rutherford in the gas discharge tube established the fact that metals on specific heat give off electrons. Therefore, the negatively charged electron particles are present in the atoms but the whole atom is electrically neutral.
Rutherford Gold Foil Experiment
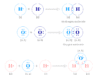
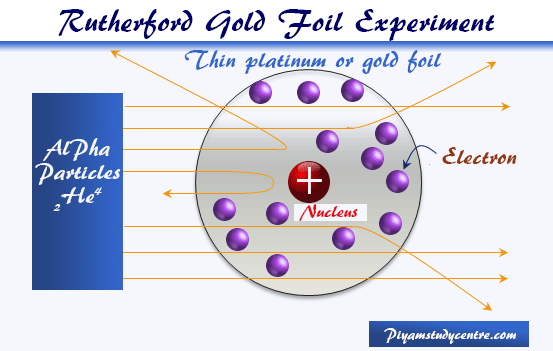
Rutherford, Geiger, and Marsden study the scattering experiment model in physics and chemistry and beam of alpha particle obtained from spontaneously radioactive decay of polonium isotopes directed to very thin platinum or gold foil.
The direction of motion of α-particles after emerging from the thin metal sheets was traced on a fluorescent screen.
Rutherford Gold Foil Experiment Observations
- Rutherford observed that the vast majority of the alpha particles passed the straight line through the gold foil model. This suggested that the chemical elements or matter consist largely of emptiness.
- But a very limited few particles (about 1 in 8000) changed their direction quite sharply. But in some cases, the deflection is more than 90°.
- Even in a few cases, the alpha particle after the encounter moved backward or reflected back. But this number increases with the change in the metal foil of the heavier periodic table elements.
Conclusion of Rutherford Model
Ernest Rutherford concluded some points from his gold foil experiment to describe the nuclear planetary model like the solar system of our universe.
- All the positive charges and the entire mass number of the atom are concentrated in a very small part of the atom. These central cores are called the atomic nucleus according to Ernest Rutherford atomic theory or model.
- The dimensions of the nucleus are negligible compared to the atom. The Radius of the atomic nucleus ∼ 10−13. Which is the same as that of an electron but the radius of an atom ∼ 10−8. Therefore Rutherford model of an atom contains a very empty structure.
- The large deflection of an alpha particle from its original path was due to Coulombic repulsion between the alpha particle and the positive charge particle of an atom. Hence entire positive charge also resides in the central part of an atom.
- Some alpha particle suffers little deflection while passing by an electron. This suggested that outside the nucleus, there are electrons that make the atom ultimately neutral.
Rutherford Discovery of Nucleus
From the above conclusions, Rutherford proposed an atomic model for learning chemistry or physics in atomic science.
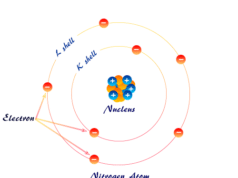
According to the Rutherford model, the entire mass of the atom is concentrated in a tiny positively charged nucleus. These electrons move around the nucleus in different orbitals constantly otherwise the electrons fall on the nucleus. An atom is divided into two parts according to Rutherford,
- The nucleus of an atom
- Extranuclear electrons
For an example of the hydrogen atom, one electron moves around the nucleus but other elements like nitrogen, oxygen, fluorine contain shielding electron.
Nucleus and Electrons
According to the Rutherford atomic model, almost the entire mass of the atom is concentrated in a very small central core called the nucleus of an atom.
Outside the nucleus, there is a definite number of electrons, called extranuclear electrons. Electrons move around the nucleus at different energy levels constantly. The extranuclear electrons contribute negligibly to the total mass of the atom. Therefore, the nucleus must carry particles that account both for the mass and positive charge of the atom.
When the electron moves in circular orbits or energy levels around the nucleus, the Coulombic attraction is balanced by the centrifugal force of attraction. Otherwise, the electrons fall on the nucleus. Therefore, Rutherford’s arrangements are quite similar to the solar system model in our environment.
Limitations of Rutherford Atomic Model
- The nuclear planetary model has limitations for conformity with the classical atomic theory of electromagnetic spectrum radiation in chemistry or physics. A moving charged particle always emits radiation with specific wavelengths. Thus the loss of kinetic energy eventually hits the nucleus of an atom.
- If the energy of the electron loses continuously in the Rutherford model, the observed hydrogen spectra should be continuous, consisting of broad bands merging one into the other. However, the Bohr model shows line spectra of the hydrogen atom.