Group 17 Elements
Group 17 Elements in Periodic Table
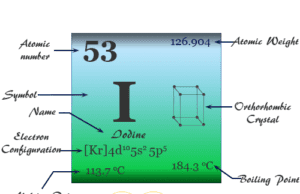
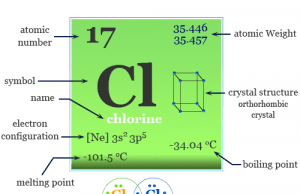
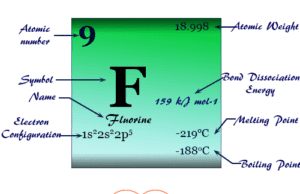
Group 17 elements also called the Halogens family in the periodic table contain the chemical element fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At) with ns2 np5 valence shell electronic configuration. These chemical elements are one electron short of octet configuration or the nearest noble gas electronic configuration. Therefore, these elements share one electron to form a covalent bond or gain one electron and form an ionic bonding. The name halogens are derived from the Greek word halo means salt and genes means producing. Therefore, these seven highly reactive non-metals in the periodic table are called “halogens”. They give salts when they react with metals. The position of halogen elements in the periodic table is given below in the picture,
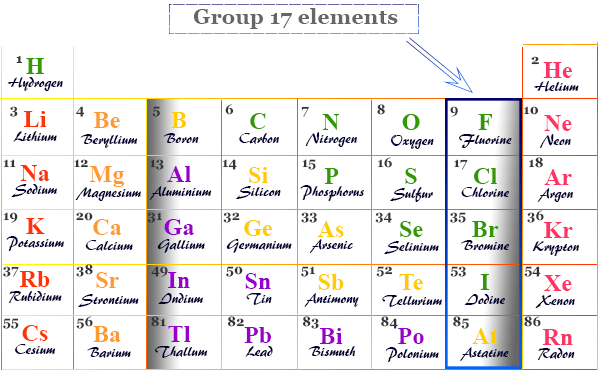
The detailed properties, use, and compounds of halogens are given below the individual topics in learning chemistry.