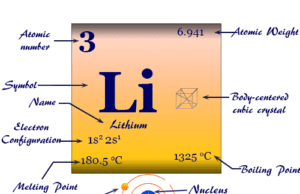
Periodic Table of Chemical Elements
Chemical elements simply called elements in the periodic table are materials that cannot be broken down or changed into another substance using any chemical process. They are the basic building blocks of matter and are present in all molecules or chemical compounds. The list of chemical elements in the periodic table now extends up to 118. The periodic table classification of these 118 chemical elements (metals, non-metals, metalloids) is based on the modern periodic law. Elements in the periodic table participate in different types of chemical bonding such as ionic bonding, covalent bonding, or metallic bonding to form substances. The name, symbol, atomic number, atomic weight, and electron per shell for all 118 elements on the periodic table are given below the picture,
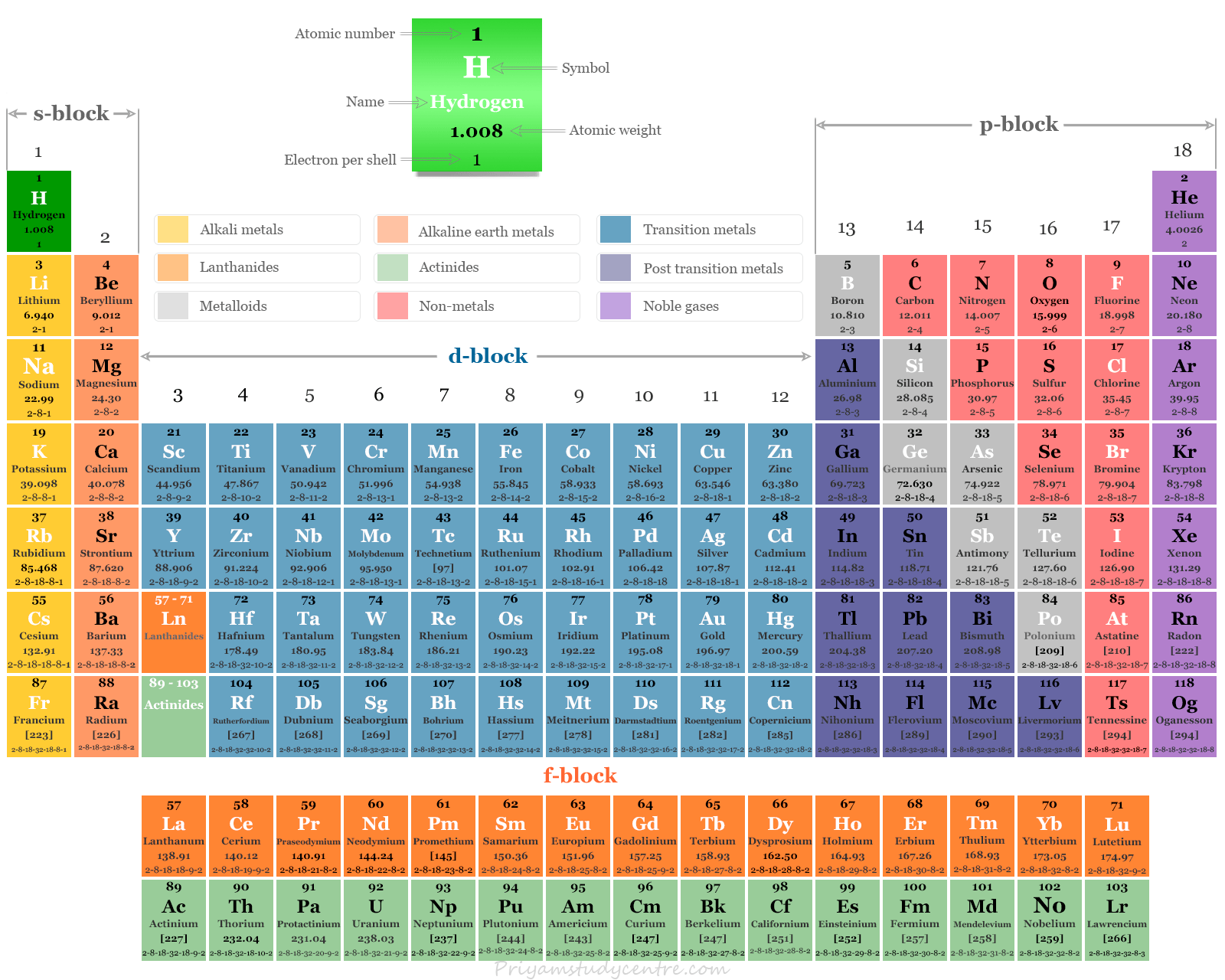
Classification of Chemical Elements
Mendeleev in 1867 first time attempted to classify chemical elements according to their atomic weight.
The post-Mendeleev developments and in order to remove the defects of the Mendeleev periodic table a number of tables have been suggested for the classification of chemical elements. All these tables are arranged in the increasing order of their atomic numbers.
Four types of chemical elements are found on the periodic table depending on the nature of the atomic orbitals in which the last electrons enter. These are,
- s-block elements
- p-block elements
- d-block elements
- f-block elements
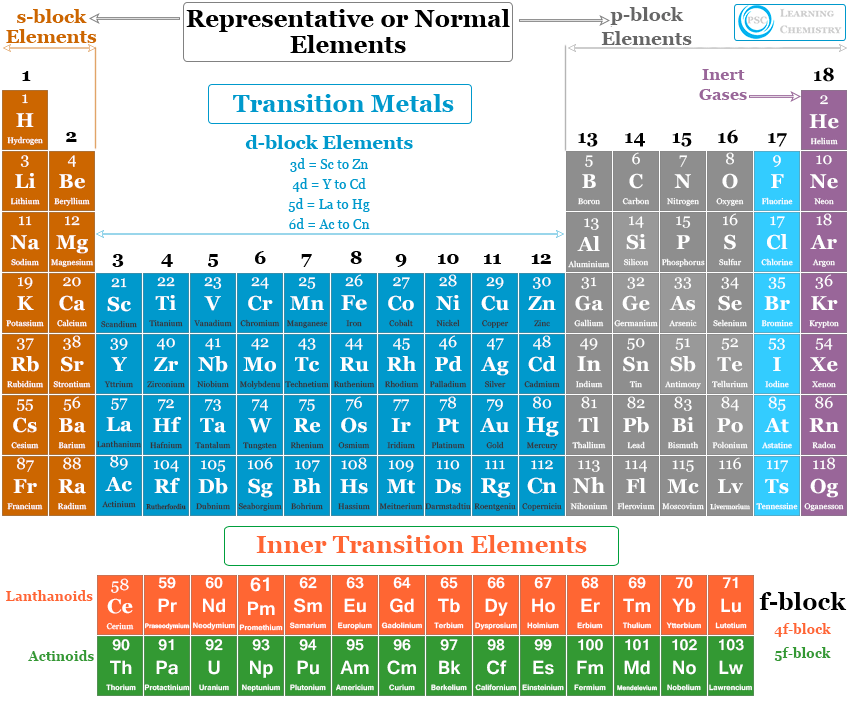
All these 118 chemical elements of the periodic table are classified into four types by Bohr on the basis of their electronic configuration. They are grouped into four classes depending on the number of incomplete shells of electrons in the atom.
- Inert gases or noble gases
- Representative or normal elements
- Transition elements
- Inner transition elements or f-block elements
Inert Gases
The lack of chemical reactivity of noble gases was the reason to call them inert. The atoms of these chemical elements have completely filled s or p-subshells. They have very little tendency to form chemical compounds due to the highly stable ns2np6 configuration or valence shell configuration (except He 1s2).
Representative or Normal Elements
The representative or normal chemical elements contain some metals, non-metals, and metalloids. They can be divided into two groups,
- s-block elements
- p-block elements
s-block Chemical Elements
The outermost electronic configuration of s-block chemical elements varies from ns1 to ns2. The members of s-block elements lie on the extreme left of the periodic table. Alkali metals and alkaline earth metals are included in this class. They are extremely electropositive in nature.
p-block Chemical Elements
The outer electronic configuration of the atoms of p-block elements varies from ns2np1 to ns2np6.
Non-metals, metalloids, and inert gases are included in these categories. These chemical elements lie on the extreme right of the periodic table.
Transition Metals
The outermost two shells in transition elements are incomplete. They are placed between s- and p-block chemical elements. Atoms of these elements have (n-1)d1 to 10nso or 1 or 2 general electronic configurations.
Inner Transition Elements
The inner transition elements are those elements whose 4f and 5f orbitals are progressively filled by electrons. Therefore, f block elements are also called inner transition chemical elements. They formed two series such as lanthanoids and actinoids.
The atoms of these chemical elements have three incomplete outer shells. These three incomplete shells are outer ns orbital, (n-1)d orbital and (n-2)f orbital. The general electronic configuration of inner transition chemical elements are (n-2)f1 to 14(n-1)d0 or 1 or 2ns2.
List of Chemical Elements
The list of 118 chemical elements with name, atomic number, symbol, atomic weight, electronic configuration, and discovery is given below the table,
| List of chemical elements |
||||||||
| Element | Atomic weight | Period | Group | Electronic configuration | Block | Discovered by | ||
| Atomic number | Symbol | Name | ||||||
| 1 | H | Hydrogen | 1.008 | 1 | 1 | 1s1 | s-block | Henry Cavendish |
| 2 | He | Helium | 4.0026 | 1 | 18 | 1s2 | p-block | Sir William Ramsay and independently by Per Teodor Cleve and Nils Abraham Langlet |
| 3 | Li | Lithium | 6.94 | 2 | 1 | [He] 2s1 | s-block | Johan August Arfvedson |
| 4 | Be | Beryllium | 9.0122 | 2 | 2 | [He] 2s2 | s-block | Nicholas Louis Vauquelin |
| 5 | B | Boron | 10.81 | 2 | 13 | [He] 2s22p1 | p-block | Louis-Josef Gay-Lussac and Louis-Jacques Thénard and Humphry Davy |
| 6 | C | Carbon | 12.011 | 2 | 14 | [He] 2s22p2 | p-block | |
| 7 | N | Nitrogen | 14.007 | 2 | 15 | [He] 2s22p3 | p-block | Daniel Rutherford |
| 8 | O | Oxygen | 15.999 | 2 | 16 | [He] 2s22p4 | p-block | Joseph Priestley and independently by Carl Wilhelm Scheele |
| 9 | F | Fluorine | 18.998 | 2 | 17 | [He] 2s22p5 | p-block | Henri Moissan |
| 10 | Ne | Neon | 20.180 | 2 | 18 | [He] 2s22p6 | p-block | Sir William Ramsay and Morris Travers |
| 11 | Na | Sodium | 22.990 | 3 | 1 | [Ne] 3s1 | s-block | Humphry Davy |
| 12 | Mg | Magnesium | 24.305 | 3 | 2 | [Ne] 3s2 | s-block | Joseph Black |
| 13 | Al | Aluminum | 26.982 | 3 | 13 | [Ne] 3s23p1 | p-block | Hans Oersted |
| 14 | Si | Silicon | 28.085 | 3 | 14 | [Ne] 3s23p2 | p-block | Jöns Jacob Berzelius |
| 15 | P | Phosphorus | 30.974 | 3 | 15 | [Ne] 3s23p3 | p-block | Hennig Brandt |
| 16 | S | Sulfur | 32.06 | 3 | 16 | [Ne] 3s23p4 | p-block | |
| 17 | Cl | Chlorine | 35.45 | 2 | 17 | [Ne] 3s23p5 | p-block | Carl Wilhelm Scheele |
| 18 | Ar | Argon | 39.95 | 3 | 18 | [Ne] 3s23p6 | p-block | Lord Rayleigh and Sir William Ramsay |
| 19 | K | Potassium | 39.098 | 4 | 1 | [Ar] 4s1 | s-block | Humphry Davy |
| 20 | Ca | Calcium | 40.078 | 4 | 2 | [Ar] 4s2 | s-block | Humphry Davy |
| 21 | Sc | Scandium | 44.956 | 4 | 3 | [Ar] 3d14s2 | d-block | Lars Frederik Nilson |
| 22 | Ti | Titanium | 47.867 | 4 | 4 | [Ar] 3d24s2 | d-block | William Gregor |
| 23 | V | Vanadium | 50.942 | 4 | 5 | [Ar] 3d34s2 | d-block | Andrés Manuel del Rio |
| 24 | Cr | Chromium | 51.996 | 4 | 6 | [Ar] 3d54s1 | d-block | Nicholas Louis Vauquelin |
| 25 | Mn | Manganese | 54.938 | 4 | 7 | [Ar] 3d54s2 | d-block | Johan Gottlieb Gahn |
| 26 | Fe | Iron | 55.845 | 4 | 8 | [Ar] 3d64s2 | d-block | |
| 27 | Co | Cobalt | 58.933 | 4 | 9 | [Ar] 3d74s2 | d-block | Georg Brandt |
| 28 | Ni | Nickel | 58.693 | 4 | 10 | [Ar] 3d84s2 | d-block | Axel Fredrik Cronstedt |
| 29 | Cu | Copper | 63.546 | 4 | 11 | [Ar] 3d104s1 | d-block | |
| 30 | Zn | Zinc | 65.38 | 4 | 12 | [Ar] 3d104s2 | d-block | Andreas Marggraf |
| 31 | Ga | Gallium | 69.723 | 4 | 13 | [Ar] 3d104s24p1 | p-block | Paul-Émile Lecoq de Boisbaudran |
| 32 | Ge | Germanium | 72.630 | 4 | 14 | [Ar] 3d104s24p2 | p-block | Clemens Winkler |
| 33 | As | Arsenic | 74.922 | 4 | 15 | [Ar] 3d104s24p3 | p-block | Albertus Magnus |
| 34 | Se | Selenium | 78.971 | 4 | 16 | [Ar] 3d104s24p4 | p-block | Jöns Jacob Berzelius |
| 35 | Br | Bromine | 79.904 | 4 | 17 | [Ar] 3d104s24p5 | p-block | Antoine-Jérôme Balard and Carl Löwig in Heidelberg |
| 36 | Kr | Krypton | 83.798 | 4 | 18 | [Ar] 3d104s24p6 | p-block | Sir William Ramsay and Morris Travers |
| 37 | Rb | Rubidium | 85.468 | 5 | 1 | [Kr] 5s1 | s-block | Gustav Kirchhoff and Robert Bunsen |
| 38 | Sr | Strontium | 87.62 | 5 | 2 | [Kr] 5s2 | s-block | Adair Crawford |
| 39 | Y | Yttrium | 88.906 | 5 | 3 | [Kr] 4d15s2 | d-block | Johan Gadolin |
| 40 | Zr | Zirconium | 91.224 | 5 | 4 | [Kr] 4d25s2 | d-block | Martin Heinrich Klaproth |
| 41 | Nb | Niobium | 92.906 | 5 | 5 | [Kr] 4d35s2 | d-block | Charles Hatchett |
| 42 | Mo | Molybdenum | 95.95 | 5 | 6 | [Kr] 4d55s1 | d-block | Peter Jacob Hjelm |
| 43 | Tc | Technetium | 97 | 5 | 7 | [Kr] 4d55s2 | d-block | Carlo Perrier and Emilio Segrè |
| 44 | Ru | Ruthenium | 101.07 | 5 | 8 | [Kr] 4d75s1 | d-block | Karl Karlovich Klaus |
| 45 | Rh | Rhodium | 102.91 | 5 | 9 | [Kr] 4d85s1 | d-block | William Hyde Wollaston |
| 46 | Pd | Palladium | 106.42 | 5 | 10 | [Kr] 4d105s0 | d-block | William Hyde Wollaston |
| 47 | Ag | Silver | 107.87 | 5 | 11 | [Kr] 4d105s1 | d-block | |
| 48 | Cd | Cadmium | 112.41 | 5 | 12 | [Kr] 4d105s2 | d-block | Friedrich Stromeyer |
| 49 | In | Indium | 114.82 | 5 | 13 | [Kr] 4d105s25p1 | p-block | Ferdinand Reich and Hieronymous Richter |
| 50 | Sn | Tin | 118.71 | 5 | 14 | [Kr] 4d105s25p2 | p-block | |
| 51 | Sb | Antimony | 121.76 | 5 | 15 | [Kr] 4d105s25p3 | p-block | |
| 52 | Te | Tellurium | 127.60 | 5 | 16 | [Kr]4d105s25p4 | p-block | Franz-Joseph Müller von Reichenstein |
| 53 | I | Iodine | 126.90 | 5 | 17 | [Kr] 4d105s25p5 | p-block | Bernard Courtois |
| 54 | Xe | Xenon | 131.29 | 5 | 18 | [Kr] 4d105s25p6 | p-block | Sir William Ramsay and Morris Travers |
| 55 | Cs | Caesium | 132.91 | 6 | 1 | [Xe] 6s1 | s-block | Gustav Kirchhoff and Robert Bunsen |
| 56 | Ba | Barium | 137.33 | 6 | 2 | [Xe] 6s2 | s-block | Humphry Davy |
| 57 | La | Lanthanum | 138.91 | 6 | 3 | [Xe] 5d16s2 | d-block | Carl Gustav Mosander |
| 58 | Ce | Cerium | 140.12 | 6 | n/a | [Xe] 4f15d16s2 | f-block | Jöns Jacob Berzelius and Wilhelm Hisinger |
| 59 | Pr | Praseodymium | 140.91 | 6 | n/a | [Xe] 4f36s2 | f-block | Carl Auer von Welsbach |
| 60 | Nd | Neodymium | 144.24 | 6 | n/a | [Xe] 4f46s2 | f-block | Carl Auer von Welsbach |
| 61 | Pm | Promethium | 145 | 6 | n/a | [Xe] 4f56s2 | f-block | Jacob .A. Marinsky, Lawrence E. Glendenin, and Charles D. Coryell |
| 62 | Sm | Samarium | 150.36 | 6 | n/a | [Xe]4f66s2 | f-block | Paul-Émile Lecoq de Boisbaudran |
| 63 | Eu | Europium | 151.96 | 6 | n/a | [Xe] 4f76s2 | f-block | Eugène-Anatole Demarçay |
| 64 | Gd | Gadolinium | 157.25 | 6 | n/a | [Xe] 4f75d16s2 | f-block | Jean Charles Galissard de Marignac |
| 65 | Tb | Terbium | 158.93 | 6 | n/a | [Xe] 4f96s2 | f-block | Carl Gustav Mosander |
| 66 | Dy | Dysprosium | 162.50 | 6 | n/a | [Xe] 4f106s2 | f-block | Paul-Émile Lecoq de Boisbaudran |
| 67 | Ho | Holmium | 164.93 | 6 | n/a | [Xe] 4f116s2 | f-block | Per Teodor Cleve and independently by Marc Delafontaine and Louis Soret |
| 68 | Er | Erbium | 167.26 | 6 | n/a | [Xe] 4f126s2 | f-block | Carl Gustav Mosander |
| 69 | Tm | Thulium | 168.93 | 6 | n/a | [Xe]4f136s2 | f-block | Per Teodor Cleve |
| 70 | Yb | Ytterbium | 173.05 | 6 | n/a | [Xe] 4f146s2 | f-block | Jean Charles Galissard de Marignac |
| 71 | Lu | Lutetium | 174.97 | 6 | n/a | [Xe] 4f145d16s2 | f-block | Georges Urbain and independently by Charles James |
| 72 | Hf | Hafnium | 178.49 | 6 | 4 | [Xe] 4f145d26s2 | d-block | George Charles de Hevesy and Dirk Coster |
| 73 | Ta | Tantalum | 180.95 | 6 | 5 | [Xe] 4f145d36s2 | d-block | Anders Gustav Ekeberg |
| 74 | W | Tungsten | 183.84 | 6 | 6 | [Xe] 4f145d46s2 | d-block | Juan and Fausto Elhuyar |
| 75 | Re | Rhenium | 186.21 | 6 | 7 | [Xe] 4f145d56s2 | d-block | Walter Noddack, Ida Tacke and Otto Berg |
| 76 | Os | Osmium | 190.23 | 6 | 8 | [Xe] 4f145d66s2 | d-block | Smithson Tennant |
| 77 | Ir | Iridium | 192.22 | 6 | 9 | [Xe] 4f145d76s2 | d-block | Smithson Tennant |
| 78 | Pt | Platinum | 195.08 | 6 | 10 | [Xe] 4f145d96s1 | d-block | |
| 79 | Au | Gold | 196.97 | 6 | 11 | [Xe] 4f145d106s1 | d-block | |
| 80 | Hg | Mercury | 200.59 | 6 | 12 | [Xe] 4f145d106s2 | d-block | |
| 81 | Tl | Thallium | 204.38 | 6 | 13 | [Xe] 4f145d106s26p1 | p-block | William Crookes |
| 82 | Pb | Lead | 207.2 | 6 | 14 | [Xe] 4f145d106s26p2 | p-block | |
| 83 | Bi | Bismuth | 208.98 | 6 | 15 | [Xe] 4f145d106s26p3 | p-block | |
| 84 | Po | Polonium | 209 | 6 | 16 | [Xe] 4f145d106s26p4 | p-block | Marie Curie |
| 85 | At | Astatine | 210 | 6 | 17 | [Xe] 4f145d106s26p5 | p-block | Dale R. Corson, Kenneth Ross MacKenzie, Emilio Segrè |
| 86 | Rn | Radon | 222 | 6 | 18 | [Xe] 4f145d106s26p6 | p-block | Friedrich Ernst Dorn |
| 87 | Fr | Francium | 223 | 7 | 1 | [Rn] 7s1 | s-block | Marguerite Perey |
| 88 | Ra | Radium | 226 | 7 | 2 | [Rn] 7s2 | s-block | Pierre and Marie Curie |
| 89 | Ac | Actinium | 227 | 7 | 3 | [Rn] 6d17s2 | d-block | Andrew Debierne |
| 90 | Th | Thorium | 232.04 | 7 | Actinides | [Rn] 6d27s2 | f-block | Jöns Jacob Berzelius |
| 91 | Pa | Protactinium | 231.04 | 7 | Actinides | [Rn] 5f26d17s2 | f-block | Kasimir Fajans and Otto Göhring |
| 92 | U | Uranium | 238.03 | 7 | Actinides | [Rn] 5f36d17s2 | f-block | Martin Heinrich Klaproth |
| 93 | Np | Neptunium | [237] | 7 | Actinides | [Rn] 5f46d17s2 | f-block | Edwin McMillan and Philip Abelson |
| 94 | Pu | Plutonium | [244] | 7 | Actinides | [Rn] 5f67s2 | f-block | Glenn Seaborg and colleagues |
| 95 | Am | Americium | [243] | 7 | Actinides | [Rn] 5f77s2 | f-block | Glenn Seaborg and colleagues |
| 96 | Cm | Curium | [247] | 7 | Actinides | [Rn] 5f76d17s2 | f-block | Glenn Seaborg and colleagues |
| 97 | Bk | Berkelium | [247] | 7 | Actinides | [Rn] 5f97s2 | f-block | Stanley Thompson, Albert Ghiorso, and Glenn Seaborg |
| 98 | Cf | Californium | [251] | 7 | Actinides | [Rn] 5f107s2 | f-block | Stanley Thompson, Kenneth Street, Jr., Albert Ghiorso, and Glenn Seaborg |
| 99 | Es | Einsteinium | [252] | 7 | Actinides | [Rn] 5f117s2 | f-block | Albert Ghiorso and colleagues |
| 100 | Fm | Fermium | [257] | 7 | Actinides | [Rn] 5f127s2 | f-block | Albert Ghiorso and colleagues |
| 101 | Md | Mendelevium | [258] | 7 | Actinides | [Rn] 5f137s2 | f-block | Albert Ghiorso and colleagues |
| 102 | No | Nobelium | [259] | 7 | Actinides | [Rn] 5f147s2 | f-block | Georgy Flerov and colleagues and independently by Albert Ghiorso and colleagues |
| 103 | Lw | Lawrencium | [266] | 7 | Actinides | [Rn] 5f147s27p1 | f-block | Georgy Flerov and colleagues and independently by Albert Ghiorso and colleagues |
| 104 | Rf | Rutherfordium | [267] | 7 | 4 | [Rn] 5f146d27s2 | d-block | Georgy Flerov and colleagues and independently by Albert Ghiorso and colleagues |
| 105 | Db | Dubnium | [268] | 7 | 5 | [Rn] 5f146d37s2 | d-block | Scientists at both Berkeley, California, USA, and Dubna, near Moscow, Russia |
| 106 | Sg | Seaborgium | [269] | 7 | 6 | [Rn] 5f146d47s2 | d-block | Albert Ghiorso and colleagues |
| 107 | Bh | Bohrium | [269] | 7 | 7 | [Rn] 5f146d57s2 | d-block | Peter Armbruster, Gottfried Münzenberg and colleagues |
| 108 | Hs | Hassium | [269] | 7 | 8 | [Rn] 5f146d67s2 | d-block | Peter Armbruster, Gottfried Münzenberg |
| 109 | Mt | Meitnerium | [278] | 7 | 9 | [Rn] 5f146d77s2 | d-block | Peter Armbruster, Gottfried Münzenberg and colleagues |
| 110 | Ds | Darmstadtium | [281] | 7 | 10 | [Rn] 5f146d97s1 | d-block | Sigurd Hofmann, Peter Armbruster and Gottfried Münzenberg |
| 111 | Rg | Roentgenium | [282] | 7 | 11 | [Rn] 5f146d107s1 | d-block | Peter Armbruster and Gottfried Münzenberg |
| 112 | Cn | Copernicium | [285] | 7 | 12 | [Rn] 5f146d107s2 | d-block | Sigurd Hofmann and colleagues |
| 113 | Nh | Nihonium | [286] | 7 | 13 | [Rn] 5f146d107s27p1 | p-block | Scientists from RIKEN in Japan |
| 114 | Fl | Flerovium | [289] | 7 | 14 | [Rn] 5f146d107s27p2 | p-block | Scientists from JINS and LLNL |
| 115 | Mc | Moscovium | [290] | 7 | 15 | [Rn] 5f146d107s27p3 | p-block | Scientists from JINS, LLNL and ORNL |
| 116 | Lv | Livermorium | [293] | 7 | 16 | [Rn] 5f146d107s27p4 | p-block | Scientists from JINS and LLNL |
| 117 | Ts | Tennessine | [294] | 7 | 17 | [Rn] 5f146d107s27p5 | p-block | Scientists from JINS, LLNL and ORNL |
| 118 | Og | Oganesson | [294] | 7 | 18 | [Rn] 5f146d107s27p6 | p-block | Scientists from JINS and LLNL |