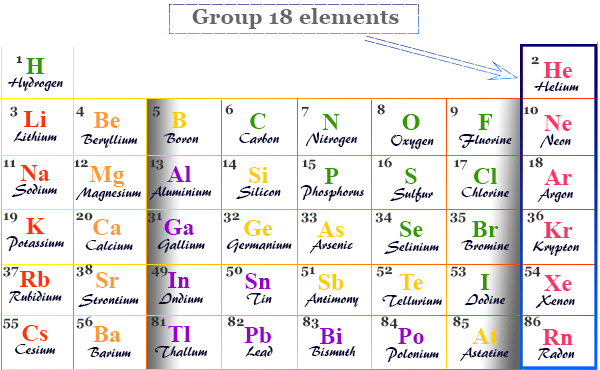
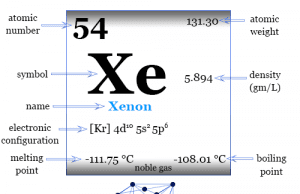
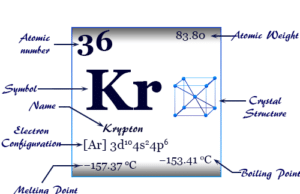
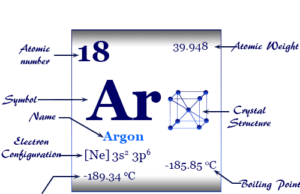
Group 18 elements
Periodic Table of Elements Group 18
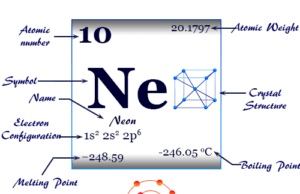
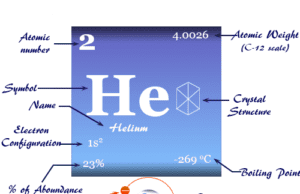
Group 18 elements also called noble gases or inert gases are the seven chemical elements of the periodic table with a closed-shell electronic configuration. The Group-18 elements like helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn), and oganesson (Og) are colorless, orderless, monoatomic gases with very low melting point and boiling point. Due to lack of reactivity, they are also called inert gas. The properties, occurrences, compounds, and uses of Group-18 elements are derived below the individual topics in learning chemistry.