Chlorine Element
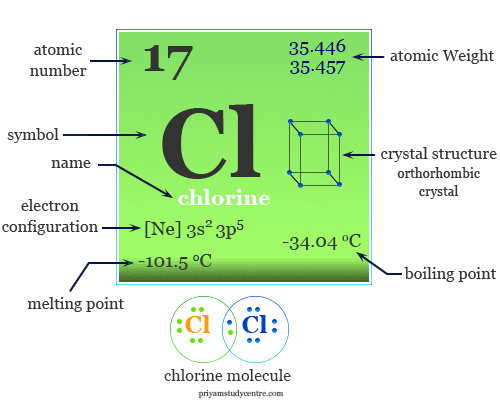
Chlorine is a nonmetal or chemical element of group-17 or the halogen family of the periodic table with the symbol Cl and atomic number 17. Molecular chlorine is a toxic, corrosive, greenish-yellow gas with the molecular formula Cl2. It is the second lightest member of the halogen family placed between fluorine and bromine. Most of the physical and chemical properties of chlorine are intermediate between fluorine and bromine. Chlorine is uses mostly for the production of bleaching agents in paper, pump, and textile industries. It is found mostly in seawater as sodium, potassium, calcium, or magnesium chloride. These chlorides have been used from the very early days of our civilization. The chemistry of chlorine follows its electronic configuration and position on the periodic table. It is a group-17 element with a 3s2 3p5 valence shell electronic configuration.
Interesting Facts About Chlorine
Due to the presence of seven valence electrons and a vacant d-orbital, it exhibits oxidation number or state −1 to +7. Therefore, it is a highly reactive oxidizing agent.
The electron affinity of chlorine is greater than fluorine but oxidizing properties are less than that of fluorine. Fluorine has the highest reduction potential of all known periodic table elements. However, electronegativity of Cl2 is the third-highest after fluorine and oxygen.
Properties of Chlorine
Some chemical properties of chlorine can be explained only by considering d-orbitals in chemical bonding. Various physical and atomic properties of chlorine are given below in the table,
| Chlorine | |||
| Symbol | Cl | ||
| Discovery | Carl Wilhelm Scheele in 1774 | ||
| Name derived from | The Greek word chloros means greenish-yellow | ||
| Molecular formula | Cl2 | ||
| Common isotopes | 17Cl35, 17Cl37 | ||
| Oxidation number or states | +7, +5, +3, +1, -1 | ||
| CAS number | 7782-50-5 | ||
| Periodic properties | |||
| Atomic number | 17 | ||
| Atomic weight | 35.48 | ||
| Electron per cell | 2, 8, 7 | ||
| Electronic Configuration | [Ne] 3s2 3p5 | ||
| Block | p-block | ||
| Group | 17 | ||
| Period | 3 | ||
| Physical properties | |||
| State at 20 °C | Gas | ||
| Melting point | −101.5 °C, −150.7 °F, 171.7 K | ||
| Boiling point | −34.04 °C, −29.27 °F, 239.11 K | ||
| Molar heat capacity | 33.949 J mol−1 K−1 | ||
| Crystal structure | orthorhombic | ||
| Density | 0.0029 g/cm3 | ||
| Critical temperature | 416.9 K | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 1.75 Å | ||
| Covalent radius | 1.00 Å | ||
| Electronegativity | 3.16 (Pauling scale) | ||
| Electron affinity | 348.575 kJ mol−1 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 1251.19 | 2297.66 | 3821.78 | |
Chemical Properties
The chemical properties and reactivity of chlorine are intermediate between halogen elements fluorine and bromine.
The bond energies trend of the halogen family does not decrease from fluorine to iodine. It is due to the small size, low electric polarization, and absence of vacant d-orbitals for chemical bonding in fluorine molecules.
The gradual decrease in bond energy from Cl2 to I2 exhibits poorer overlap with increasing overlapping orbitals. It shows that chlorine chemistry is different from fluorine. It exhibits positive oxidation states.
Chlorine in Water
Chlorine is dissolved in many organic hydrocarbons but sparingly soluble in water, during which they disproportionate into HCl or HOCl.
The solid hydrate (Cl2, 8H2O) actually contains the water molecule. The water molecule solid hydrate is trapped by the hydrogen bonding network.
Chlorine on the Periodic Table
Chlorine is found in period 4 and group 17 in the periodic table. It is a p-block eminent which lies between fluorine and bromine.
Who Discovered Chlorine?
Scientific research and uses of different rock salts (common salt or sodium chloride) in our environment are associated with the very early history of our incident civilization.
Around 1630, chlorine gas was first developed in the chemical process but was not recognized as a chemical element.
The Swedish chemist Carl Wilhelm Scheele in 1774 discovered a greenish-yellow gas by reaction of magnesium with hydrochloric acid but he failed to recognize the chlorine gas.
In 1810, English chemist Humphry Davy established that it is an element. He suggested the name of chlorine gas from the Greek word chloros, meaning yellowish green.
The term halogen was given by J.S.C. Schweigger for its ability to form salts with metal atoms.
Where is Chlorine Found?
Chlorine was found mainly in sodium chloride deposited in seawater and obtained by evaporation of seawater. The concentration of sodium chloride in seawater is about 2 percent.
In different types of landlocked seas like the Caspian Sea and the Dead Sea, salt concentration increases up to 30 percent.
It was also found in brine wells and rock salt deposits. A small amount is found in the blood and milk of living organisms.
It consists of two common isotopes like 35Cl (76 percent) and 37Cl (24.5 percent).
Production Process
Laboratory Preparation of Chlorine Gas
Chlorine gas is generally prepared in the laboratory by oxidation of hydrochloric acid with manganese dioxide (MnO2) or potassium permanganate (KMnO4).
MnO2 + 4 HCl → MnCl2 + Cl2 + 2 H2O2
KMnO4 + 16 HCl → 2 MnCl2 + 2 KCl + 8 H2O + 5 Cl2
Industrial Production of Chlorine
On the industry or technical scale, chlorine gas is obtained during the electrolysis of sodium chloride or in an aqueous solution (brine). The metallic sodium, hydrogen gas, and sodium hydroxide are the by-products which are the most valuable materials.
In this process, we use a mercury cathode, the metal being gradually discharged owing to alarming mercury pollution through the water and energy efficient process. Due to this possibility, we use traditional diaphragms for the separation of electrodes by the membrane.
Production of Vinyl Chloride
The old Deacon process of oxidation of hydrogen chloride by air in the presence of CuCl2 chemical catalyst at 450 °C is also triad again for industrial production of Cl2 gas.
The chemical equilibrium is shifted to the right by converting the Cl2 to dichloroethane uses for the production of vinyl chloride.
Uses of Chlorine
About 25 million tonnes of chlorine gas are produced annually in the world by most industrially developed countries. The main uses of chlorine are,
- Chlorine gas is used as a bleaching agent in the paper, pulp, and textile industries.
- It is also used as a disinfectant in water supply or treatment of sewage water pollution.
- Chlorine is used for manufacturing hydrochloric acid, and other inorganic compounds like NaClO3, NaOCl, Al2Cl6, SOCl2. etc.
- Due to its high oxidizing potential, elemental chlorine uses in commercial bleaches, disinfectants, and many chemical processes in the chemical industry.
- About 70 percent of chlorine is used for the production of chlorinated organic compounds like ethyl dichloride and polyvinyl chloride (PVC). It is also used in intermediate reactions for the production of plastic.
- A high-concentration chorine molecule is a toxic poisonous gas for living organisms. It is used as a poisonous gas weapon in World War I.
Chemical Compounds
Metal Halides
It reacts with almost all the elements in the periodic table except helium, neon, and argon. Therefore, chlorine forms a wide range of halides. The compounds are formed by simple ionic or covalent bonding or molecular species or polymeric species.
In addition to such binary halides, it forms many oxohalides, hydroxohalides, and complex halides formed by several elements. AlCl3, TiCl4, PCl3, SbCl3, FeCl3, and PbCl4 are the most common examples of metal or nonmetal halides.
Hydrogen Chloride
Hydrogen chloride or hydrochloric acid is an important compound of chlorine known to the chemist from the earliest time. It is used largely in the chemical industry.
Hydrogen chloride is conventionally prepared by the reaction of sodium chloride with concentrated sulfuric acid or burning hydrogen in Cl2 gas.
The aqueous solution of the hydrogen chloride conducts electricity owing to extensive ionization. Due to weak bond dissociation energy, hydrogen chloride is a strong acid in an aqueous solution (approximately pKa values = 7).
Oxides of Chlorine
The oxides of halogens are unstable chemical compounds. The higher oxides are beings rather stable than the lower ones. The stability of oxides of I2 is greater than that of Cl2 while the oxides of Br2 are the least stable.
Cl2O, Cl2O3, ClO2, Cl2O4, Cl2O6, and ClO7 are examples of oxides of chlorine formed by different types of chemical reactions. Most of them are soluble only in the aqueous solution or as salts. Chlorine also forms different types of oxoacids in oxidation states +1, +3, +5, and +7.