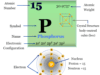
Fluorine Element
Fluorine (chemical symbol F), chemical formula F2, atomic number 9 is the most electronegative and most chemically reactive member of the halogen family or Group 17 of the periodic table element. Due to high reactivity and the small size, fluorine combines directly with metal, almost all nonmetal, and noble gases. The reactions with many elements are vigorous and sometimes explosive. The exceptionally high reactivity of fluorine is assigned due to low F-F bond energy ( 159 kJ mol−1). The reactivity is gradually decreasing in other members of the group (chlorine, bromine, and iodine).
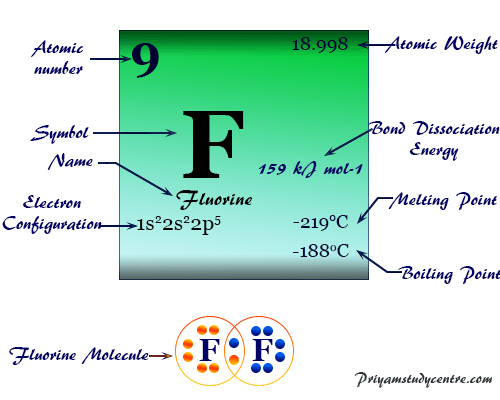
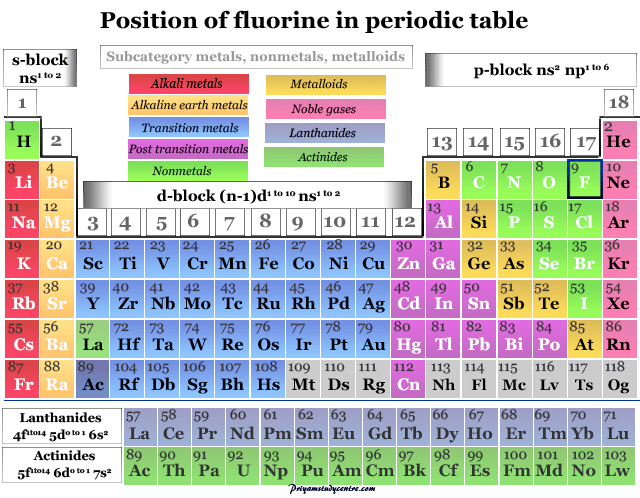
Fluorine on the Periodic Table
It is placed in group 17 (VIIB) on the periodic table with the halogen family. The chemistry and periodic table position of halogens follow their electronic configuration. All the elements have seven electrons in the valence orbitals.
Who Discovered Fluorine?
The name of the chemical element fluorine originated from the mineral fluorspar (CaF2) suggested by French physicist André-Marie Ampère in a letter to Davy (1812).
In 1771 the Swedish chemist Carl Wilhelm Scheele discovered the corrosive compound hydrofluoric acid (HF). He heated fluorspar with concentrated sulfuric acid in a glass retort. However, it could not be used for the isolation of fluorine due to several difficulties.
For more than 150 years, all the chemical preparation methods have failed to produce F2. However, the success of discovery came in 1986 by American chemist Karl O. Christe through the reaction of K2MnF6 and antimony pentafluoride (SbF5). Both chemical compounds are obtained by using hydrogen fluoride.
Occurrence of Fluorine
None of the halogens are found free in nature owing to their high reactivity. It occurs in the earth’s crust to the extent of 0.065 percent (544 ppm; thirteenth in abundance). Therefore, the abundance of the element is higher than that of chlorine (0.055 percent, 126 ppm, twentieth in abundance).
The most common minerals found in the earth’s environment concentrated with fluorine elements are fluorite or fluorspar (CaF2), cryolite (Na3AlF6), and fluorapatite[3Ca3(PO4)2, CaF2].
Where is Fluorine Found?
The principal fluorine-containing minerals (fluorspar) are found in different parts of the world like India, Illinois, Kentucky, Derbyshire, southern Germany, the south of France, and Russia.
Greenland is also the chief source of mineral-like cryolite (Na3AlF6). In India, fluorite occurs mostly in Chhattisgarh (Drug, Raipur, and Jabalpur) and in Rajasthan (Jaipur, Ajmer, and Kishangarh).
Production Process
Preparation of Fluorine by Electrolysis
French chemist Henri Moissan 1886 prepared fluorine by electrolysis of potassium fluoride (KF) with anhydrous hydrofluoric acid in a U-tube of platinum with a platinum-iridium electrode.
He received the Nobel Prize in chemistry in 1906 for the production of chemical elements. Due to its toxic characteristics, the progress of fluorine chemistry is very slow.
Fluorine from Hydrogen Fluoride
Anhydrous hydrofluoric acid is a nonconductor of electricity while an aqueous solution produces ozonized oxygen on electrolysis. No chemical oxidizing agent was able to oxidize a fluoride compound or hydrofluoric acid.
The decomposition of fluoride compounds gives fluorine gas. However, the process is unlikely from the thermodynamic point of view since the compounds have high lattice or bond energy.
It is now prepared by electrolysis molten mixture of potassium fluoride and hydrofluoric acid in a mild cell acting as the cathode and the anode is the central rod of nongraphitic carbon separated from the cathode by the porous diaphragm.
Physical Properties
Fluorine is a faint yellow-greenish gas at an ordinary temperature that turns into a yellow liquid after cooling. It has only one stable isotope 19F or F-19. In the vapour state, it normally exists as a diatomic molecule (F2) with a melting point of −219 °C and a boiling point of −188 °C.
F2 molecule is formed by weak Van der Waals attraction. In the solid phase, it forms layers of the crystal lattice.
| Fluorine | ||||
| Symbol | F | |||
| Discovery | Henri Moissan in 1886 | |||
| Name derived from | Latin word fluere, meaning to flow | |||
| Common isotope | 19F | |||
| Crystal structure | Cubic crystal lattice | |||
| Periodic properties | ||||
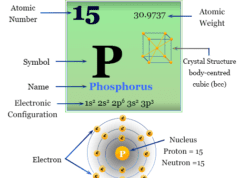
| Atomic number | 9 | |||
| Electron per shell | 2, 7 | |||
| Atomic weight | 18.998 | |||
| Electronic configuration | [He] 2s2 2p5 | |||
| Group | 17 (Halogen) | |||
| Period | 2 | |||
| Block | p-block | |||
| Physical properties | ||||
| State at 20 °C | Gas | |||
| Melting point | −219.67°C, −363.41°F, 53.48 K | |||
| Boiling Point | −188.11°C, −306.6°F, 85.04 K | |||
| Density | 0.001553 g cm−3 | |||
| Chemical properties | ||||
| Atomic radius (non-bonded) | 1.47 Å | |||
| Covalent radius | 0.60 Å | |||
| common oxidation number or states | −1 | |||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd | |
| 1681.04 | 3374.17 | 6050.44 | ||
| Electron affinity | 328.165 kJ mol−1 | |||
| Electronegativity | 3.98 (Pauling scale) | |||
| Molar heat capacity |
Cp: 31 J mol−1 K−1 at 21.1 °C Cv: 23 J mol−1 K−1 at 21.1 °C |
|||
| CAS number | 7782-41-4 | |||
Chemical Properties
Fluorine is the most electronegative chemical element in the periodic table. Therefore, it always assigns a −1 oxidation number and introduces polarity in hydrogen fluoride molecules. The hydrogen fluoride molecules are associated via intermolecular hydrogen bonding. The chemistry and periodic table position of fluorine can be described by its electronic configuration which is one electron short of the next noble gas.
Facts About Fluorine
Some of the interesting facts about higher halogens can be explained only when considering the participation of d-orbitals in chemical bonding. Some facts about fluorine are explained below,
- The noble gas electronic structure of the fluorine atom is achieved in two ways, by gaining one electron from reactive surroundings to form an ion or by forming a shared pair in covalent bonding. It has the tendency to accept one electron due to high electron affinity and ionization energy.
- It brings about the maximum coordination number of other elements due to the small size of the atom and extremely high electronegativity.
Both these facts permit a maximum number of small fluorine atoms without accumulation of excessive charge density on central atoms such as SF6, OsF6, [SiF6]2−, IF7, etc.
What is Fluorine Used for?
- At least 10,000 tonnes of fluorine gas (F2) is produced annually over the world which is used in the manufacture of UF6 in nuclear power stations, in making SF6 dielectrics and fluorinating agents like ClF3, BrF3, etc.
- Tungsten (W) and Rhenium (Re) fluorinated to volatile WF6 and ReF6 required in vapour deposition of metals on machine components
- Synthetic cryolite is also a very important chemical compound of F2 and it has wide industrial applications.
- The chemical compound fluorspar also uses as a flux in metallurgy.
- The chemical compounds of fluorine like hydrogen fluoride (HF), chlorofluorocarbon (CFC), and boron trifluoride (BF3) are used mostly in the industry and synthesis of organic compounds.