Ionic Bonding Definition
Ionic bonding or ionic bond is the electrostatic force that binds together oppositely charged ions formed by the transfer of electron or electrons from an electropositive metal to an electronegative non-metal atom. We know that sodium and chlorine react vigorously to yield crystalline solid sodium chloride. The crystallographic study suggests that there is no single sodium chloride molecule in the NaCl crystal lattice. Each sodium ion is surrounded by six chloride ions in a non-ending pattern by electrostatic forces of attraction or ionic bonding. The formation of an ionic compound like sodium chloride by chemical bonding between the sodium atom and chlorine atom is given below the picture,
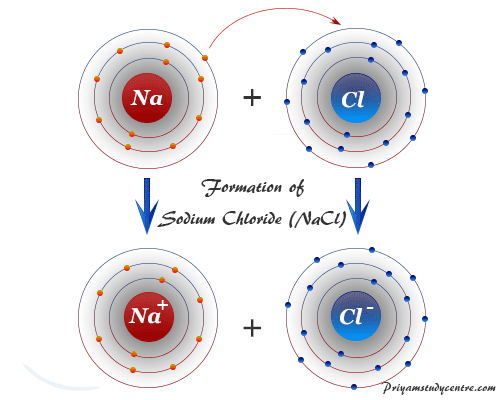
Formation of Ionic Bond
- Every halogen atom has seven electrons in its outermost orbital. Therefore, they need one electron to attain a stable electronic configuration of the nearest noble gas.
- On the other hand, every alkali metal has one electron in the outermost orbital. Therefore, they try to attain a stable electronic configuration by losing one electron.
They are combined by the electrostatic force of attraction to form ionic compounds like sodium chloride (NaCl), potassium chloride (KCl), sodium bromide (NaBr), potassium bromide (KBr), etc.
Examples of Ionic Bonding
Similar types of ionic bonds are also formed in molecules like calcium chloride (CaCl2), calcium oxide (CaO), magnesium sulfide (MgS), magnesium nitride (Mg3N2), and aluminum oxide (Al2O3).
For example, in calcium chloride, calcium oxide, and magnesium sulfide two electron transfers occur for the formation of ionic bonding. In such a combination, every participant atom would attain a stable inert gas electronic structure.
The chemical bond with the transfer of electrons between the participant’s atom is called an ionic bond.
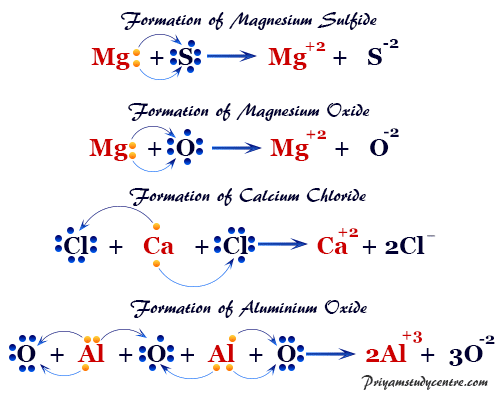
Properties of Ionic Bonding
Attainment of stable noble gas electronic configuration is the clue of formation ionic and covalent bonding or chemical reactions. Such a conclusion also helps us to predict the chemical elements which most likely to form ionic compounds.
- The elements which have low ionization energy are the best candidates for forming positive ions or cations.
- Similarly, the elements that have high electron affinity are most likely to form negative ions or anions.
- The large size of the metal atom or ion is favored for the formation of ionic compounds. With the increasing atomic size ionization energy decreases.
- Again the small size and low charge on the anions will be favored for the formation of ionic compounds. These factors generally increase the electron affinity or electronegativity of the atom.
Unexpected result is sometimes observed. For example, the second electron affinity of oxygen is negative but it forms ionic bonding or compounds with alkalis or alkaline earth metals.