Covalent Bond Examples
Covalent bond or covalent bonding may be defined as a chemical bond that is formed by the sharing of one or more electron pairs with similar or dissimilar atoms. Each atom of periodic table elements contributes one electron to form an electron pair in this type of chemical bonding. American scientist G. N Lewis first points out that, it was possible for forming the covalent bond in a molecule to attain an inert gas structure without any transference of electrons or ionic bonds. The compounds like hydrogen (H2), chlorine (Cl2), oxygen (O2), water (H2O), and hydrocarbons (methane, ethane, propane) are examples of chemical compounds formed by the covalent bond. All the participating covalent bonding atoms complete their octet structure or adopt the nearest noble gas configuration.
Ionic and Covalent Bonding
It is easy to understand the formation of the ionic bond by the definition but much more difficult to understand covalent bonds in chemistry. An ionic bond is formed by the transference of electron or electrons from one atom to another.
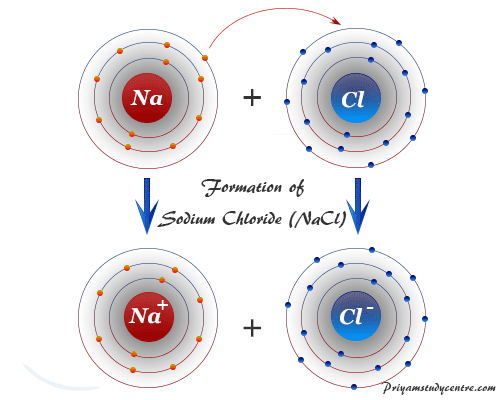
In 1919 American scientist G. N Lewis defined a covalent bond as the forces holding together atoms through the sharing of electrons.
Lewis Structure for Covalent Compounds
Lewis’s theory clearly explains the formation of covalent bonds through the sharing of electrons and most types of chemical properties of covalent bonding. However, Lewis’s theory does not provide the mechanism of sharing electrons leading to the stability of the covalent bonding.
The mechanism of sharing electrons is given by quantum mechanics.
How to Draw Lewis Structures?
- Counting the outer quantum shell electrons or valence electrons of the combing atoms. If species are cation then eliminate the electron. But for anion add one electron for each negative charge.
- Put the larger or less electronegative atom as the central atom.
- Arrange the electron pairs keeping in mind to allow for octets for the atoms.
- For the first period, chemical elements in the periodic table like boron, carbon, nitrogen, oxygen, and fluorine form the covalent bond by sharing one or more electrons.
- However, hydrogen has one electron in the valence shell. Therefore, hydrogen may not be suitable for the octet rule.
- PCl5 and SF5 are other examples in learning chemistry, where the octet rule breaks down.
The Octet Rule
When two atoms form covalent compounds, they attain a noble gas configuration in their valence shell. This is known as the octet rule. In a hydrogen molecule, each of the hydrogen atoms shares one electron to attain a 1s2 configuration.
Exceptions to the Octet Rule
In BeCl2, BF3, PCl5, ClF3, ICl3, SF6, and NO are examples of molecules in which the octet rule breaks down. The atoms have electrons either less than eight (incomplete octet) or more than eight (expansion of octet)
- In BCl2, BF3, and NO, the central beryllium, boron, and nitrogen atoms are surrounded by four, six, and seven electrons in their valence shell. These are examples of incomplete octet rules.
- In PCl5, ClF5, ICl3, and SF6 molecules, the central phosphorus, chlorine, iodine, or sulfur atom is surrounded by 10 or 12 electrons in their valence shell. These are examples of the expansion of the octet.
Types of Covalent Bond
According to the number of electron pairs, the covalent bond may have three types
- Single covalent bond
- Double covalent bond
- Triple covalent bond
Single Bonds
Single covalent bonds are formed by the sharing of only one electron pair between the bonded atoms. A chlorine atom has seven electrons in the outermost quantum energy level.
According to Lewis, two chlorine atoms unite to form the covalent chlorine molecule. By covalent chemical bonding, each atom contributes electrons to make a pair that may be shared by both atoms.
Multiple Bonds
Lewis’s representation extended to cover multiple covalent molecular bonds also. Double and triple covalent bonds are collectively called multiple bonds.
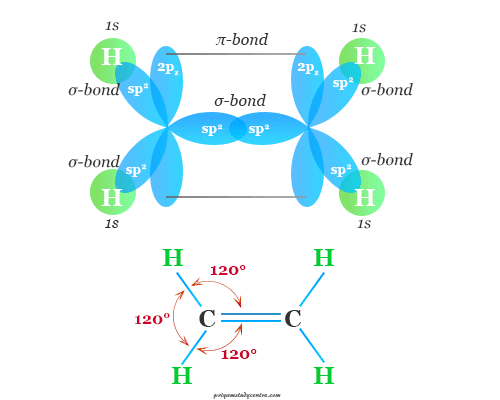
These types of bonds are formed when the atoms bonded together by sharing two or three electron pairs respectively.
For example, bonding in oxygen, carbon dioxide, and ethylene molecules is formed by multiple covalent bonding. In the acetylene molecule, we can find the triple covalent bond.
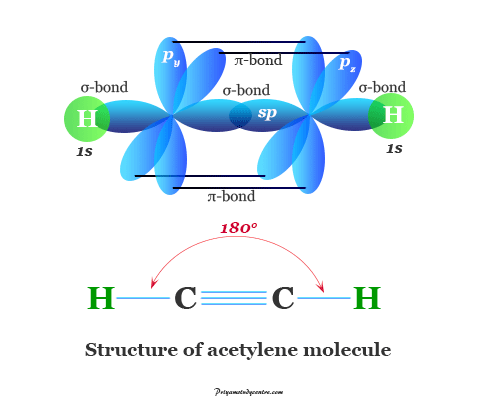
Example of Covalent Compounds
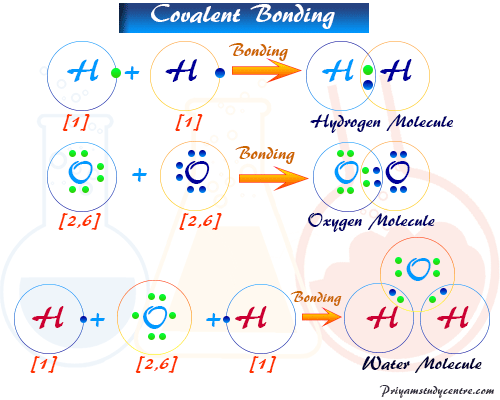
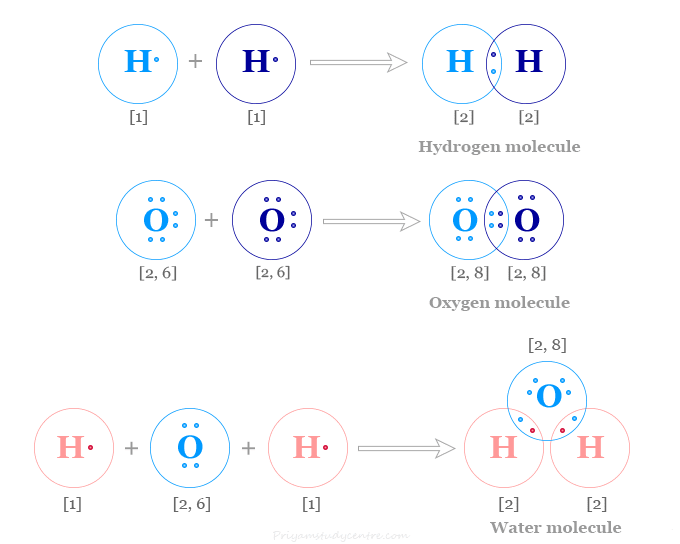
The electronic configuration of the hydrogen atom, 1s1. Therefore two hydrogen atoms covalent bonding by sharing one electron to form a hydrogen molecule. In the above picture, we represent covalent bonding in hydrogen, oxygen, and a water molecule.
Properties of Covalent Bond
- Covalent compounds are formed by the sharing of electrons between atoms. There are no ion formations and atoms are held by weak Van der Waals forces.
- They are generally soluble in organic solvents like alcohol, ether, or carbon tetrachloride.
- Covalent compounds are solids, liquids, or gases.
- Their melting and boiling points are low.
- These are soft, easily fusible, and volatile.
- A covalent bonding has directional properties. Therefore, they are formed by isomerism and stereoisomers.
Polar Covalent Bond Definition
The binary homonuclear covalent molecule has to be nonpolar. Since the bonding elements possess the same electronegativity. In such cases, the bonding electrons are shared equally by two atoms.
Heteronuclear polar diatomic molecules are polar due to differences in their electronegativities. However, electronegativity is the not only part of polarity. The polarity of a covalent bond or molecule is also decided by the composition and geometry of the molecule.