Radon Gas
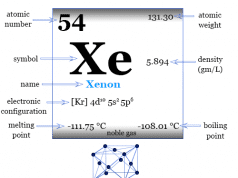
Radon is a chemical element or noble gas or Group 18 element of the periodic table with the symbol Rn and atomic number 86. It was discovered by Ernest Rutherford and Soddy in 1899 from the radioactive decay of substances. It is a colorless, odorless, tasteless, and heavy radioactive element obtained from radium-226 by alpha particle emission. Naturally, a small quantity of radon is formed during the radioactivity or chain reaction of uranium and thorium. It is colorless at ambient temperature but when cooled below its melting point, it emits brilliant yellow phosphorescence. The color changes to orange-red when cooled with liquid nitrogen. At room temperature, the density and solubility of radon in water are the highest among all noble gases like He, Ne, Kr, and Xe.
Where is Radon Found?
It is found in rock, soil, and natural gases. The burning of fossil fuels like coke or natural gas releases radon gas.
Rn-222 is the longest-lived isotope of the element with a half-life of 3.8 days. It is a member of all three natural radioactive series.
Radon gas moves from different sources to air, groundwater, and surface water. It is coming to our homes through the water supply.
Radon on the Periodic Table
Prior to 1960 helium, neon, argon, krypton, xenon, and radon were known as inert or rare gases. Now it is called noble gas and placed on group-18 or group-0 of the periodic table. This gas was not known in Mendeleev’s time.
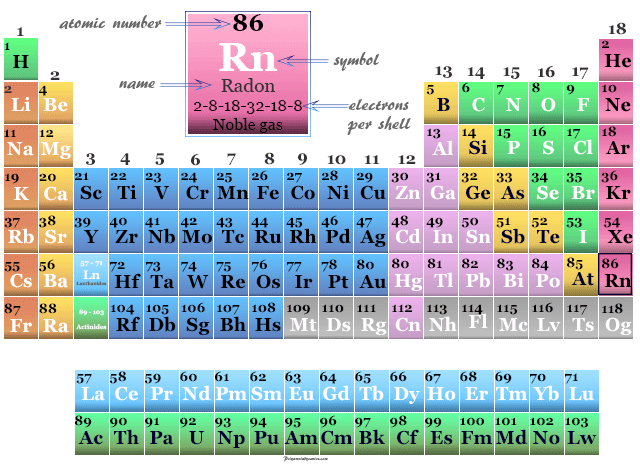
When it was discovered and characterized, due to extreme inertness, the gas is placed extremely right on the periodic table.
Radon Isotopes
Radon-222 is the longest-lived radioactive isotope of the element having a half-life of 3.8 days. Due to the very short-lived, the study of the element is extremely difficult.
It has no stable isotopes but it formed thirty-nine radioactive isotopes with atomic mass ranging from 193 to 231.
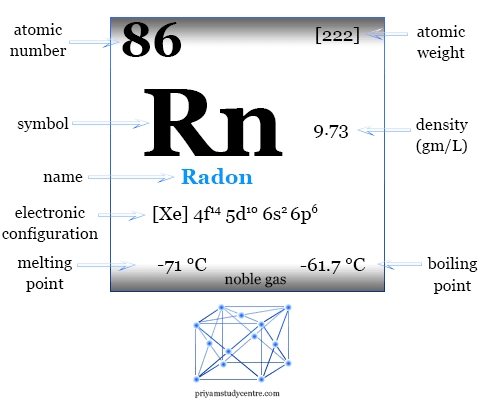
Properties of Radon
| Radon | |||
| Symbol | Rn | ||
| Discovery | Friedrich Ernst Dorn in 1900 | ||
| Name derived from | Radium, it was first detected from the radioactive decay of radium | ||
| Common isotope | 211Rn, 220Rn, 222Rn | ||
| Oxidation states | 0, +2, +6 | ||
| CAS number | 10043-92-2 | ||
| Periodic properties | |||
| Atomic number | 86 | ||
| Relative atomic mass | [222] | ||
| Electron per cell | 2, 8, 18, 32, 18, 8 | ||
| Electronic Configuration | [Xe] 4f14 5d10 6s2 6p6 | ||
| Block | p-block | ||
| Group | 18 | ||
| Period | 6 | ||
| Physical properties | |||
| State at 20 °C | Gas | ||
| Melting point | −71 °C, −96 °F, 202 K | ||
| Boiling point | −61.7 °C, −79.1 °F, 211.5 K | ||
| Molar heat capacity | 20.786 J mol−1 K−1 | ||
| Crystal structure | face-centered cubic (fcc) | ||
| Density | 0.009074 g/cm3 | ||
| Critical temperature and pressure | 377 K and 6.28 MPa | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.20 Å | ||
| Covalent radius | 1.46 Å | ||
| Electronegativity | unknown | ||
| Electron affinity | unknown | ||
| Ionization energy (kJ/mol) | 1037.07 | ||
Chemical Properties
Like other noble gases, it is also a member of zero valence elements which makes it chemically inert. The ionization energy of radon is slightly lower than xenon. The fact suggests that Rn is more reactive than Xe.
Due to the small half-life, the study of the chemical properties of radon is very difficult. It is naturally used as a natural tracer for chemical analysis. It is volatile above −80 °C but the compounds are not volatile at the same condition.
Production Process
It is produced directly by alpha-ray decay of an aqueous solution of radium chloride.
88Ra226 → 86Rn222 + 2He4
The gas is largely diluted by ozone, hydrogen, and oxygen produced from the alpha-irradiation of water. The mixture of gases passed over heated copper and copper (II) oxide and finally dried with phosphorus pentoxide to obtain pure radon.
Facts About Radon Element
Inspired by the development of xenon chemistry, it is logical to form the rich chemistry of radon from energy consideration. But due to the very short life and small availability of the element in the earth’s atmosphere, its study is very difficult in science.
One g of Ra-226 produces only 1g of Ra. Therefore, the study of the element is restricted to tracer studies.
Chemical Compounds
Radon reacts with fluorine gas at room temperature or liquid fluorine at −196 °C, the reaction vessel retains some gamma-activity after the volatile substance is pumped out.
It suggests that an ionic chemical bond might be formed in ionic fluoride like Rn+F− or (RnF+)F−. The fluorine molecule is reduced by hydrogen gas at 500 °C to set free the element from the fluoride compound. It is also oxidized by several interhalogen cations to give Rn+ or RnF+ which may be retained by ion exchange resin.
Similar oxidation may be carried out in liquid chlorine trifluoride (ClF3) or bromine trifluoride (BrF3). On electrolysis, the radon is found to move to a positive electrode or cathode, which suggests that it formed cationic species on electrolysis.
Effects of Radon Gas
- Changes in the concentration of radon in groundwater are the symbol to predict the Earthquake in our environment.
- It occurs in nature as a gas that affects human health to causes lung cancer due to the formation of free radicals.
- Radon is a known pollutant when it is emitted from renewable energy sources like geothermal power stations but it does not have more impact on our earth’s environment or human health.
It causes lung cancer by breathing. According to the United States Environmental Pollution Agency, it is the second material that causes lung cancer after cigarette smoking.
Uses of Radon
- It is prepared commercially for use in radiation therapy. It is used in radiation therapy to destroy carcinogenic cancer cells.
- Radon is also used as a nucleotide material in the reactor of nuclear power plants.
- The electromagnetic spectrum radiation from radioactive radon is used in testing metal casting and industrial radiography.