Xenon Gas
Xenon is a chemical element or inert or noble gas of Group 18 of the periodic table with the symbol Xe and atomic number 54. It is found in trace amounts in the earth’s environment. Due to filled valence orbital and high ionization energy, the oxidation number or state is zero, but with fluorine, xenon also shows a higher oxidation state. It is a colorless, dense, odorless, tasteless, monoatomic gas that forms true chemical compounds with fluorine. Xenon is used in flash or arc lamps and as an anesthetic in medicine. It is also used as a starter gas in high-pressure sodium lamps. The gas is mostly used in light-emitting devices like flash lamps, photographic flashes, high-pressure arc lamps, and shorter-lived carbon arc lamps.
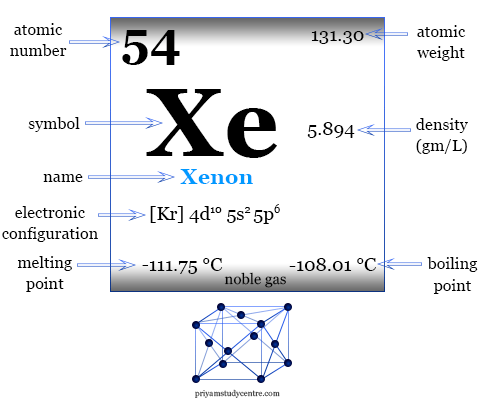
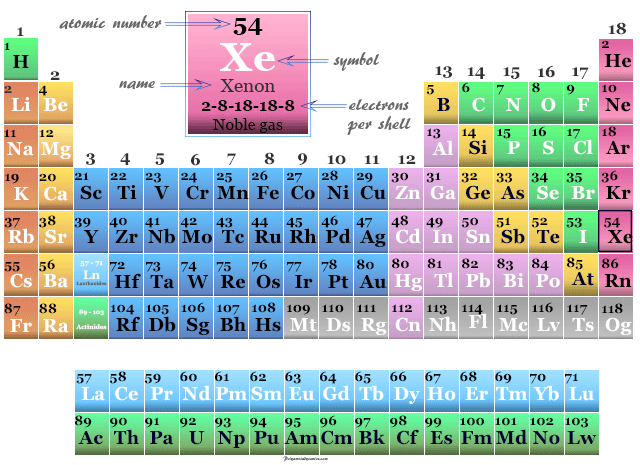
Xenon in the Periodic Table
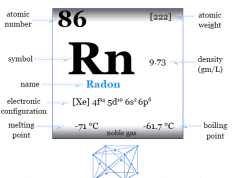
Xenon is placed in group 18 and period 5 in the periodic table. It is a noble gas that lies between krypton and radon.
Who Discovered Xenon?
A heavy and extremely rare gas, xenon was discovered by the British chemists Sir William Ramsay and Morris W. Travers. The name of the gas was derived from the Greek letter Xenos meaning strange or foreign.
Where is Xenon Found?
It occurs in the earth’s atmosphere to the extent of 0.00001 percent by volume. Xenon is manufactured on a small scale by the fractional distillation of liquid air. Xenon is rare in the earth’s atmosphere and more expensive than other noble gases like helium, neon, argon, and krypton.
Properties of Xenon
The rare gas, xenon has valence shell electron configuration [Kr] 4d10 5s2 4p6, and a heat capacity ratio (Cp/Cv) close to 1.66. In solid-state, xenon forms a face-centered cubic crystal lattice, and the cubic close-packed structure is the most space-economizing.
| Xenon | |||
| Symbol | Xe | ||
| Discovery | Sir William Ramsay and Morris Travers in 1898 | ||
| Name derived from | The Greek word xenos means stranger | ||
| Common isotope | 54Xe132 | ||
| Oxidation states | 0, 6, 4, 2 | ||
| CAS number | 7440-63-3 | ||
| Periodic properties | |||
| Atomic number | 54 | ||
| Relative atomic mass | 131.293 | ||
| Electron per cell | 2, 8, 18, 18, 8 | ||
| Electronic Configuration | [Kr] 4d10 5s2 5p6 | ||
| Block | p-block | ||
| Group | 18 | ||
| Period | 5 | ||
| Physical properties | |||
| State at 20 °C | Gas | ||
| Melting point | −111.75 °C, −169.15 °F, 161.40 K | ||
| Boiling point | −108.10 °C, −162.58 °F, 165.05 K | ||
| Molar heat capacity | 21.01 J mol−1 K−1 | ||
| Crystal structure | face-centered cubic (fcc) | ||
| Density | 0.005366 g/cm3 | ||
| Critical temperature | 289.733 K | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.16 Å | ||
| Covalent radius | 1.36 Å | ||
| Electronegativity | 2.60 (Pauling scale) | ||
| Electron affinity | unknown | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 1170.35 | 2023.78 | 3099.40 | |
Isotopes
Natural xenon is a mixture of nine stable isotopes. The atomic mass and natural abundance of these xenon isotopes are given below in the table,
| Isotope | Atomic mass | Natural abundance (%) |
| 124Xe | 123.906 | 0.0952 |
| 126Xe | 125.904 | 0.089 |
| 128Xe | 127.904 | 1.9102 |
| 129Xe | 128.905 | 26.4006 |
| 130Xe | 129.904 | 4.071 |
| 131Xe | 130.905 | 21.2324 |
| 132Xe | 131.904 | 26.9086 |
| 134Xe | 133.905 | 10.4357 |
| 136Xe | 135.907 | 8.8573 |
Different types of artificial radioactive isotopes are obtained by the nuclear fission reaction whose life is very small.
Xe-135 is produced in the reactor of the nuclear power plant by nuclear transmutation of uranium. Xe-135 has a huge cross-section for thermal neutrons since it can be considered a neutron absorber in a nuclear reaction or chain reaction.
A huge number of radioactive isotopes of xenon are also released from the nuclear reactor during the nuclear fission reaction.
Uses of Xenon

Xenon is rare in the earth’s atmosphere and more expensive than other noble gases like helium, neon, argon, and krypton.
- The gas is used in specific light-emitting devices like flash lamps, photographic flashes, high-pressure arc lamps, and shorter-lived carbon arc lamps.
- It is a starter gas in high-pressure sodium lamps.
- Xenon isotopes are powerful tools for studying solar renewable energy systems and nuclear magnetic resonance spectroscopy.
Facts About Xenon
The chemistry of xenon and other noble gases like He, Ne, Kr, and Rn is different from other periodic table elements due to their stable valence shell electron configuration. They cannot participate in covalent bonding due to very high electron promotional energy.
The formation of chemical bonding to form an ionic compound like Xe+F− is similarly difficult due to very high ionization energy. Xe2+ ion is stable in a vacuum with a bond order of 0.5 and usually high bond energy.
Prior to 1962, no true chemical compound of xenon is known. Clathrates or cage compounds are formed with para-quinol and water.
Chemical Compounds
As early as 1933, Linus Pauling attempted to make the chemical compound XeF6 but failed until 1962. In 1962, British chemist Neil Bartlett produced the first yellow-orange solid xenon compound that contains a mixture of [XeF+][PtF6−], [XeF+][Pt2F11−].
He observed that PtF6 changes color on exposure to air. It was subsequently established that PtF6 is a very strong oxidizing agent capable of oxidizing oxygen and xenon. This was the beginning of noble gas chemistry.
Very soon different types of xenon compounds, including halides, oxides, oxofluorides, oxo salts, and numerous covalent derivatives are discovered.
Xenon Fluoride
Xenon fluorides like XeF2, XeF4, and XeF6 are thermodynamically stable compounds. They are obtained by heating Xe and fluorine in a sealed nickel vessel. Halides such as XeCl2, XeClF, XeBr2, and XeCl4 are thermodynamically unstable. Therefore, they have been detected only in small amounts.
All three xenon fluorides are colorless volatile crystalline solids but in the liquid or gas phase, XeF6 shows yellow color. All the fluorides are readily hydrolyzed to form Xe or XeO3. The formation of XeO3 is highly explosive which caused many accidents during the study of XeF4 and XeF6.
All three fluorides are strong oxidizing and fluorinating agents, reactivity increasing from XeF2 to XeF6. Therefore, XeF6 combines with hydrogen and attacks glass at ordinary temperature but others attack by heating.
XeF2 is used as a fluorinating agent in alky or aryl hydrocarbon synthesis. It also acts as an oxidizer of silver (I) to silver (II) and chromium (III) to chromium (IV) and BrO3− to BrO4−.
Xenon fluorides react with fluoride ion acceptors like pentafluoride (MF5), where M = arsenic, antimony, bismuth, niobium, tantalum, ruthenium, osmium, iridium, and platinum. Here halides act as a base for strong Lewis acid like MF5.
Xenon Oxyfluorides
XeOF4 (melting point −46 °C) and XeO2F2 (melting point 31 °C) are the colorless crystalline oxyfluoride of xenon.
- The square pyramidal, XeOF4 is formed in the controlled hydrolysis of XeF6.
- The oxyfluoride XeO2F2 is the same structural formula, formed by the reaction of XeO3 with XeOF4 or XeF6.
Other oxofluorides of xenon like XeOF2 and XeOF3 are unstable and formed in a low-temperature matrix by reacting Xe with OF2 or by hydrolysis of XeF4 at low temperatures.
XeOF2 disproportionates in the presence of CsF to give anion [XeO2F3]−. Stable white salt of [XeO3F]− is formed by reacting aqueous XeO3 with CsF or KF.
Oxides, Xenates, and Perxenate
The xenon trioxide, XeO3 is the end product of hydrolysis of XeF6. The violence of the reaction is controlled when sweeping XeF6 vapor with dry nitrogen into the water. XeO3 crystals consist of trigonal pyramidal units with xenon atoms at the apex.
The tetraoxide, XeO4 is formed by the action of concentrated sulfuric acid on sodium and barium perxenates. However, a perxenate of lithium and potassium is formed by the disproportionation of XeO3 and XeF6 in the respective alkaline solution.
The dichloride, XeCl2 is not formed under usual conditions. However, it may be formed when the Xe and chlorine mixture passes through a microwave discharge and rapidly cools. XeCl2, XeBr2, and XeCl4 have also been detected by Mossbauer spectroscopy in the beta-particle decay.
A chemical compound (BXeF3) containing a boron xenon bond is formed during the reaction of O2BF4 and xenon at −100 °C. It contains a planner structure in which one fluorine atom in XeF2 is replaced by a BF2 unit.