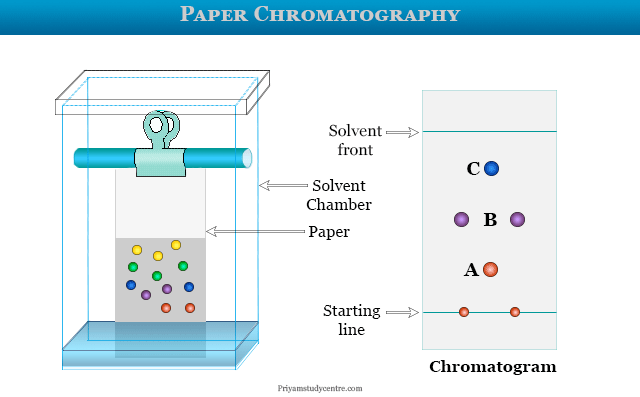
Paper Chromatography Experiments
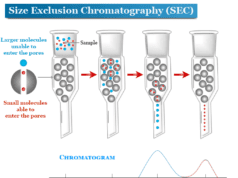
Paper chromatography in analytical chemistry is a technique that we used for the identification and separation of coloured samples. In paper chromatography experiments, a mixture of substances is located and identified by the flow of a mixture of two solvents which is immiscible or partially miscible on specially designed Whatman filter paper. The solvent rises up on paper owing to capillary action. Paper chromatography experiment is used mainly for the separation or identification of amino acids, dyes, or sugars. Presently, the paper chromatography procedure is used as a teaching tool in labs due to the discovery of other chromatography methods such as thin-layer chromatography, ion-exchange chromatography.
In 1944 Consden, Gordon, and Martin first introduced the paper chromatography technique.
Paper Chromatography Principle
- In paper chromatography experiments, a drop of mixed solution is placed near one short end of rectangular filter paper by a microsyringe or capillary tube. The spot on the paper is allowed to dry.
- The spotted filter paper is suspended vertically in the solvent chamber where the spot just touches solvent levels. Simply, the filter paper below the spot dipped into the solvent.
- When the solvent front moves upward and passes the spot, it carries the different components upwards. The rates of upward capacity depend on the nature of substances, the polarity of the solvents, and the size of the molecules.
- The solvent front is allowed to move upward until it travels 3/4 of the height of the filter paper.
What is Rf Value?
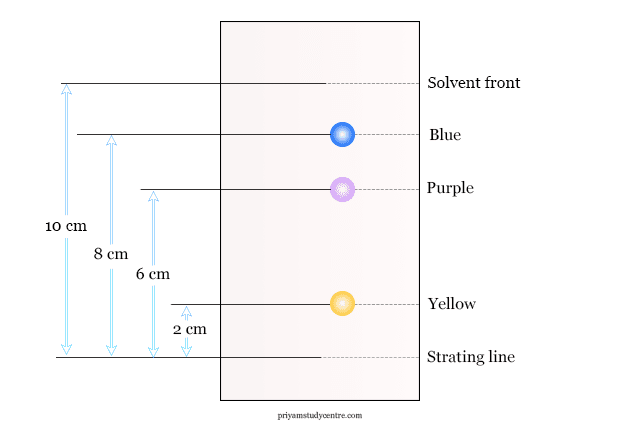
The relative rate of movement of solute and solvent is called the Rf value. It is the ratio of the distance traveled by the sample component and the distance traveled by the solvent.
Rf value is constant for a given substance under given conditions. Therefore, various components can be identified by their Rf value in paper chromatography. Rf value has no unit but varies with the change of solvent.
Experiment with Amino Acids
A paper chromatography experiment is used for the identification or separation of individual amino acids from a mixture of amino acids.
Material Required in Paper Chromatography
- Whatman filter paper strip.
- A mixture of unknown amino acids is dissolved in 10 ml of water.
- Solution of glycine: 5 mg of glycine in 1 ml of water.
- Ninhydrin spray: 200 mg of ninhydrin is dissolved in 99 ml of n-butanol and 1 ml of acetic acid.
- Solvent: n-butanol, acetic acid, and water = 80ml, 20 ml, and 100ml.
Paper Chromatography Procedure
A line parallel to the short end of the chromatography paper sheet is drowned by a pencil about 10 cm apart. Two points are marked on the Whatman filter paper strip.
The mixture of amino acids is spotted on one mark with no more than 5 mm diameter. Similarly, the second mark is spotted by the standard solution of glycine. The spot is allowed to dry.
The developing solvent is placed in a clean dry glass chamber of a paper chromatographic instrument. The glass chamber is covered with a glass plate having a hole in the middle.
The paper strip hangs from a wire hook dipped into the solution where the spots are just above the solvent front. The solvent rises owing to capillary action on the paper strip. When it almost reaches the point of suspension from the wire hook, the paper strip is carefully taken out from the chamber.
Paper Chromatography Experiment Result
The position of the solvent front on the paper strip is marked. The paper strip is allowed to dry and the ninhydrin reagent is spread lightly but uniformly. The strip is then heated in an oven at 105 °C for five minutes and the position of the spots is marked.
- The yellow spot is identified as proline.
- The spot which moves parallel to the spot of glycine is identified as glycine.
- The other blush purple spot is phenyl aniline.
Paper Chromatography Types
There are three main types of paper chromatography such as descending, ascending, and circular type.
- In descending type, the liquid is allowed to run down the paper under gravity.
- In ascending type, the solvent runs in an upward direction by capillary action.
- The third is called radial development or circular chromatography where circular paper is used.
Uses of Paper Chromatography
It is used mainly for the identification of substances such as amino acids, sugars, dyes, etc. The separations by the paper chromatography principle are possible because of the continuous partitioning of substances between the aqueous phase and organic mobile phase running down the paper.
Solute migration is initiated from a small narrow spot. Surface tension is the driving force for capillary action. Therefore, a liquid-liquid partition is predominant in the mechanism of paper chromatography.
The practical application of the paper chromatography technique for the identification of sugars such as glucose, fructose, and sucrose is given below,
Material Required
- Whatman No. 1 filter paper strip.
- Solvent: n-butyl alcohol, glacial acetic acid, and water.
- Spring reagent: Aniline oxalate, 0.093 g of aniline is dissolved in 50 ml of 0.95% of ethanol and added 50 ml 0.2(M) aqueous oxalic acid. The mixture is mixed uniformly. The solutions filtrate if necessary.
- Supplied solution: Aqueous solution of glucose, fructose, and sucrose which contain 10 mg of each substance dissolved in 4 ml of water.
Procedure
Setting the ascending paper chromatography instrument. Identify the spot developed in the chromatogram.
Experiment result
| Compound | Colur of the spot in the chromatogram | Rf value |
| Sucrose | Yellow | 0.08 |
| Glucose | Yellow | 0.17 |
| Fructose | Yellow | 0.25 |