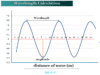
Chromatography techniques
Chromatography techniques in analytical chemistry were applied to coloured components at the initial development of the chromatography principle. The name chromatography originated from colour but today it is applied also to colourless compounds. Adsorption, partition, ion exchange, exclusion, affinity, and electrophoresis are the main types of chromatography techniques that we used widely in our daily life or laboratory. The principle of the chromatography technique has been discovered accidentally when a drop of ink suddenly falls on a blotting paper.
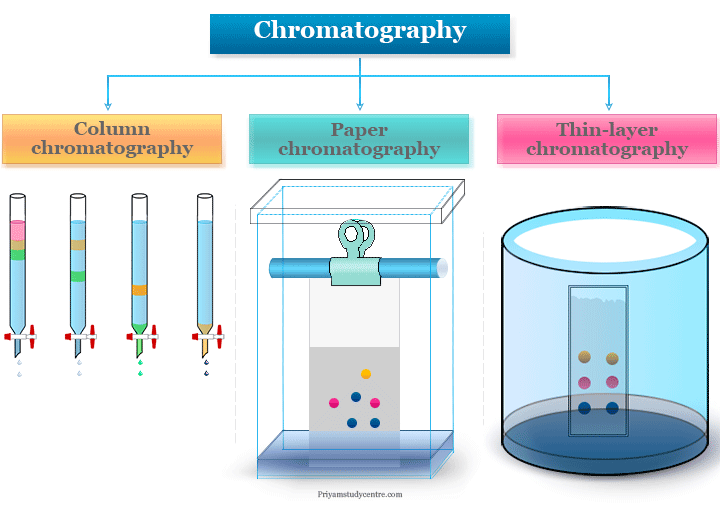
Chromatography components are distributed into two phases like stationary phase and mobile phase. The stationary phase is a column, plate, or paper but the mobile phase is liquid or gas in chromatography.
Chromatography principle
Chromatography is based on the principle where molecules are transferred between the mobile phase and the stationary phase. It occurs due to the absorbance or partition of molecules present in the mixture which we want to separate.
The rate of travel of individual solute molecules through a column or thin layer is not uniform. It is directly related to the partition of the molecules between the mobile phase and stationary phase.
Stationary phase in chromatography
The stationary phase may be a column, plate, or paper in chromatographic technique. It is a solid phase or a liquid phase coated on the surface of a solid phase.
Depending upon the nature of the stationary phase, three chromatographic techniques have been used widely in the laboratory. These techniques are,
- Liquid-solid absorption or column chromatography (LSC)
- Paper chromatography (PC)
- Thin-layer chromatography (TLC)
Mobile phase in chromatography
The mobile phase (liquid or gas) moves through the stationary phase when different components are separated due to different rates of migration.
- If the mobile phase is liquid, it is called liquid chromatography (LC).
- If the mobile phase is gas then it is called gas chromatography (GC).
The migration of molecules depends on their affinity to the stationary and mobile phases. Therefore, the components which are attached less by the stationary phase will move faster in mobile phases.
Types of chromatography
Separation techniques cannot contain one type of property. We classify columnar techniques like distillation, solvent extraction, and ion exchange in one class. They can also be classified according to the migration ions.
Based on physical properties and phase separation, chromatography techniques are the following types,
| Separation techniques | |
| Basis of separation | Technique |
| Volatility | Distillation |
| Partition coefficient | Gas chromatography |
| Paper chromatography | |
| Thin-layer chromatography | |
| Exchange of ions | Cation exchange |
| Anion exchange | |
| Surface activity | Molecular sieve |
| Gel filtration | |
| Ion or size exclusion | |
| Electromigration | Electrophoresis |
| Dialysis | |
| Reserve osmosis (RO) | |
The classification of different types of chromatographic techniques or methods is very simple according to the mobile and stationary phases. The mobile phase can be gas or liquid while the stationary phase can be liquid or solid. Therefore we have liquid-liquid, liquid-solid, and gas-solid combinations.
The modern types of chromatographic methods can be classified in the following ways given below the table,
| Chromatography technique | ||
| Technique | Method | Abbreviation |
| Adsorption chromatography | Columnar method | LC |
| Gas solid chromatography | GSC | |
| Partition chromatography | Liquid-liquid partition | LLPC |
| Paper chromatography | PC | |
| Thin-layer chromatography | TLC | |
| Reverse phase partition | RPPC | |
| Ion exchange chromatography | Cation exchange | CEC |
| Anion exchange | AEC | |
| Inorganic exchanger | IE | |
| Liquid exchanger | LIE | |
| Electrophoresis | Zone electrophoresis | ZE |
| Boundary layer method | BLE | |
| Curtain electrophoresis | CC | |
| Capillary electrophoresis | CZE | |
The high-performance liquid chromatography (HPLC) technique is highly instrumental. Therefore, we cannot conclude HPLC in this classification. A short description of the most familiar types of chromatographic techniques or methods is given below the articles,
Adsorption chromatography
The adsorption chromatography technique is based upon the exploitation of the difference in absorbability of solute to the stationary phase which is packed with a column
Various fatty acids can be easily separated by the adsorption technique. The separation of noble gases on active charcoal can be done by gas-solid adsorption chromatography.
Partition chromatography
It is the largest technique of chromatography. All the techniques like LLPC, PC, TLC, GLC, and RPPC have belonged under the partitioning technique. In liquid-liquid partition chromatography, separation is affected by the distribution of the samples between a stationary phase and a mobile phase which has limited miscibility.
A solution of a substance is shaken with an immiscible solvent and the solute is distributed between the two phases. When equilibrium is reached, the partition coefficient
(Kd) = concentration of solute in solvent A/concentration of solute in solvent B.
Ion exchange resin method
The ion exchange resin method is based upon differences in the exchange potential between various ions to ion exchange resin packed in a column.
The synthetic cation exchanger has been used for the separation of the lanthanides and rare earth elements. The ion-exchange chromatography technique is also used for the separation of organic molecules such as amino acids, proteins, DNA, RNA, etc.
It was discovered by Thomas and Way in England. The exchange of sodium and calcium in soil to form sodium or calcium aluminum silicate indicate the phenomena of ion exchange.
We widely used the ion-exchange technique in several operations in our daily life such as water softening, metal separation, radiochemical separation, etc.
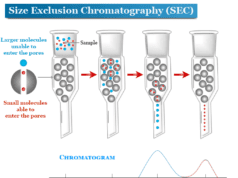
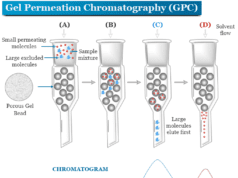
Exclusion techniques
The separation technique by exclusion chromatography is based upon the exploitation of the size or molecular geometry of the molecules. By the difference in size, certain particles move faster while other particles move slower in a column packed with gels. Therefore, it creates a differential migration on the column packed with gels.
The exclusion technique has been divided into the following categories,
- Gel permeation or gel filtration technique
- Ion exclusion and ion retardation technique
- Inorganic molecular sieves
Electrophoresis technique
Electrophoresis is a technique of separation of constituents under the influence of an electric current due to differences in the migration rate of ions.
Under the influence of direct current, the ions which have better ionic mobility move faster than lower mobility. These phenomena are used for the separation of ions by the electrophoresis technique. Several methods under the electrophoresis technique do not contain columns, plates, or capillary tubes.
The methods which used columns, plates, or capillary tubes are called electrophotographic methods. The electrophoresis technique can be classified into the following categories,
- Free boundary electrophoresis
- Capillary zone electrophoresis
- Continuous electrophoresis
Affinity chromatography
We used the affinity chromatography technique for the purification of enzymes, hormones, antibodies, DNA, RNA, proteins, and amino acids. It is also called bispecific adsorption or bioaffinity chromatography.
The formation of a specific dissociable complex with biomolecules such as enzymes, hormones, antibodies, DNA, RNA, proteins, and amino acids is the basis of the affinity technique.
We used the affinity technique for the isolation and purification of biological macromolecules. For example, isolation, and analysis of insoluble stretch substances, enzymes, proteins, and antibiotics are carried out by affinity chromatography.