Ethanol (Ethyl Alcohol)
Ethanol or ethyl alcohol is a colorless, inflammable simple alcohol or organic compound used as a fuel and solvent in chemistry. It is also called grain alcohol or drinking alcohol. The molecular formula of ethanol is C2H6O but it can be written as C2H5OH or CH3CH2OH or EtOH. Ethanol and methanol have resembled each other very closely but they can be distinguished by oxidation reactions. Ethyl alcohol gives acetic acid on oxidation but methanol gives formic acid on oxidation. These two acids readily distinguish each other. Formic acid reduces ammonical silver nitrate and the salts of many heavy metals but acetic acid does not participate in this reaction. Ethyl alcohol is miscible with water in all proportions and also miscible with most organic solvents.
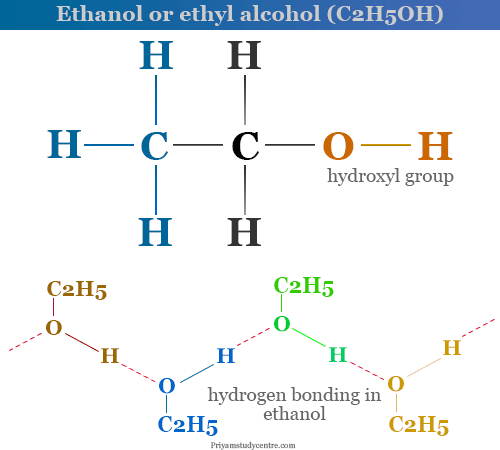
Ethyl Alcohol Structural Formula
Analysis and molecular weight determinations show that the molecular structure of ethyl alcohol is C2H6O. The carbon atom in the ethanol molecule is quadrivalent or sp3 hybridized while the oxygen atom is bivalent and hydrogen is univalent.
- Only one hydrogen atom in ethyl alcohol is replaced by a sodium or potassium atom. This fact indicates that the combination of one hydrogen atom in an ethanol molecule differs from the other five hydrogens.
- When it is treated with hydrochloric acid or phosphorus pentachloride, only one oxygen and one hydrogen atom are replaced by a chlorine atom to give ethyl chloride. This suggests the presence of one hydroxyl group in ethanol molecules.
- When ethyl chloride is hydrolyzed with dilute alkali, ethanol is obtained. The reaction also indicates the presence of a hydroxyl group in the ethanol molecule.
Properties of Ethanol
Ethanol is a volatile, colorless, slight odor liquid that is miscible with water in all proportions due to hydrogen bonding with water molecules. Ethanol is less volatile due to association through hydrogen bonding extending over a chain of molecules. It burns in the air to form luminous blue fame.
| Ethanol | |
| IUPAC name | Ethanol |
| Other names | absolute alcohol drinking alcohol ethylic alcohol ethyl alcohol ethyl hydroxide ethylol grain alcohol methylcarbinol |
| Chemical formula | C2H6O |
| CAS Number | 64-17-5 |
| Molar mass | 46.069 g mol−1 |
| Appearance | Colourless liquid |
| Density | 0.789 g/cm3 at 20 °C |
| Boiling point | 78.1 °C |
| Melting point | −114.14 °C |
| Dipole moment | 1.69 Debye |
| Solubility | Missable in water and most organic solvents |
| Acidity in water | 15.9 |
| Viscosity | 1.2 mPa s at 20 °C |
Production of Ethanol
Ethylene to Ethyl Alcohol
Industrially, ethyl alcohol is prepared from ethylene which is obtained from creaking petroleum.
Ethylene is absorbed in concentrated 98 percent sulfuric acid at 75 to 80 °C under pressure to produce ethylene hydrogen sulfate and ethyl sulfate.
C2H4 + H2SO4 → C2H5OSO3H
C2H5OSO3H + C2H4 → (C2H5O)2SO2
The reaction mixture is diluted by an equal volume of water and wormed to produce ethyl alcohol together with diethyl ether. Ether is separating from the reaction mixture.
C2H5SO3H + H2O → C2H5OH + H2SO4
(C2H5O)2SO2 + 2 H2O → 2 C2H5OH + H2SO4
(C2H5O)2SO2 + C2H5OH → (C2H5)2O + C2H5SO3H
It is also manufactured by direct hydration of ethylene with steam under pressure in the presence of a suitable chemical catalyst like phosphoric acid. A small amount of ether is formed as a by-product of this method.
Fermentation Process
Fermentation is the metabolic process that produces organic substances by the action of enzymes. The earliest method for the production of ethanol is fermentation. It is still used for the production of alcoholic beverages like beer, whisky, brandy, etc.
Stretch is the starting material for the production of ethyl alcohol. Wheat, barley, and potato are the common sources of starch.
- The food grains like wheat or barley are mashed with hot water and heated with malt at 50 °C for one hour. Malt contains the enzyme diastase which hydrolyzes starch into sugar.
- The liquid is cooled to 30 °C and fermented with yeast for 1 to 3 days.
- Yeast contains different types of enzymes. Among these maltase converts maltose into glucose.
C12H22O11 + H2O → C6H12O6 - Another enzyme zymase converts glucose into ethanol.
C6H12O6 → 2 C2H5OH + 2 CO2
The carbon dioxide obtained by the fermentation process is recovered as a by-product.
The fermentation liquor contains 6 to 10 percent of ethanol and some other compounds like acetaldehyde, n-propyl alcohol, n-butyl alcohol, isobutyl alcohol, n-amyl alcohol, isoamyl alcohol, and active amyl alcohol.
Industrial alcohol is an ordinary rectified spirit. Absolute alcohol is 99.5 percent ethanol obtained from rectified spirit.
Uses of Ethanol
- Ethanol is used widely for the production of alcoholic beverages (beer, whisky, and brandy), medicine, and flavoring or coloring of foods.
- It is used for manufacturing vinegar.
- It is used in hand sanitizer due to its antibacterial and anti-fungal effects.
- It is also used as an antidote for ethylene glycol or methanol poisoning.
- Ethanol is used for the preparation of organic compounds like ester, chloral, chloroform, etc.
- Ethanol is mixed with methanol to produce methylated spirit which is commonly used as a solvent and cooking fuel for camping and fondue burners.
- It is used as a fuel or fuel additive in different types of engines like cars, motors, or rockets.
- Ethanol is used as a solvent for the production of gums, resins, paints, and varnishes.