Solvent Definition in Chemistry
A solvent in chemistry is a chemical substance in which other types of materials dissolve to form a solution. It is the major component of a chemical solution. Commonly, solvents are liquid but they may also exist as a solid, gas, or supercritical fluid. The solvent is broadly classified into two types polar and non-polar. Most common examples of liquid solvents may include water, ammonia, benzene, ethanol, methanol, acetone, etc. These inorganic and organic solvents are used to dissolve the solutes to form a homogeneous mixture. Water is the most common polar solvent which we used for dissolving various types of chemical substances and organic molecules such as ions and proteins present in a cell.
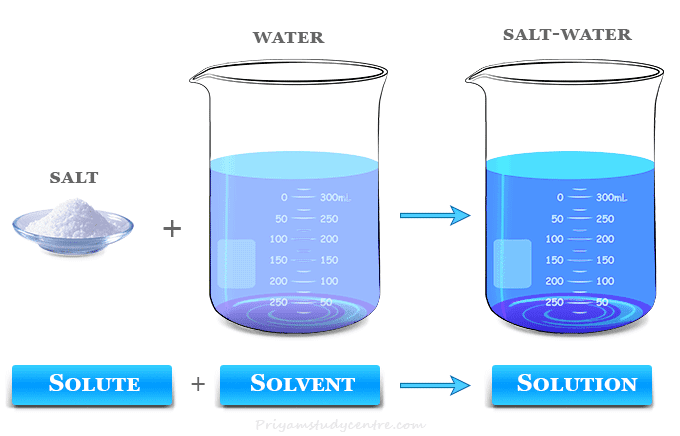
The solvent may be toxic or non-toxic in nature. Water and benzene are commonly used solvents in many industrial and household products due to their low toxicity. In recent years, the green chemistry movement used to design, develop, and implement non-toxic and sustainable chemical solvents.
Non Aqueous Solution
A non-aqueous solution is a solution in which the solvent is any liquid other than water. Ammonia, hydrogen fluoride, sulfuric acid, and hydrogen cyanide are common examples of protic nonaqueous solvents. Prominent members of aprotic nonaqueous solvents are sulfur dioxide, sulfuryl chloride, dinitrogen tetroxide, antimony trichloride, and bromine trifluoride.
Types of Solvent
Different types of solvents may be classified in a number of ways depending on their physical and chemical properties. Commonly, solvents are classified into two categories,
- Ionizing (polar or ionic) solvents
- Non-ionizing (non-polar or non-ionic) solvents
The dielectric constant of the solvent provides the rough measurement of solvent polarity.
- For example, strong polar solvents like water, ammonia, hydrogen fluoride, and sulfur dioxide have very high dielectric constant.
- While non-polar solvents like benzene and carbon tetrachloride have a very low dielectric constant (less than 15).
Polar Solvents
Ionizing solvents are polar or ionic in nature. Hence, they can dissolve ionic compounds and initiate ionic reactions. Polar solvents exist as ions in their pure state. Therefore, they are the weak conductors of electricity. These types of solvents have a high value of dielectric constants and a strong tendency to form associated structures.
Many polar solvents like water, alcohols, and other hydroxyl-containing substances such as acetic acid have the ability to form hydrogen bonding with solute molecules.
A polar solvent has a tendency to participate in self-ionization reactions. The self-ionization reaction of water, ammonia, and sulfur dioxide are,
H2O + H2O → H3O+ + OH−
NH3 + NH3 → NH4+ + NH2−
SO2 + SO2 → SO+2 + SO3−2
Non-polar Solvents
Non-polar solvents are non-ionic in nature. Therefore, they can dissolve only natural or non-polar compounds and do not initiate ionic reactions.
Non-polar solvents have low dielectric constants. They have very little associating and solvating tendency between solvents and solutes. Common examples of non-polar solvents are organic hydrocarbons, benzene, toluene, carbon tetrachloride, etc.
On the basis of proton donner and proton acceptor properties, the solvents may be classified in three ways,
- Protic solvent
- Aprotic or non-protic solvent
- Amphoteric solvent
Protic Solvent
A protic solvent has a hydrogen atom in its formula. In other words, it is a solvent that contains a labile H+ ion. They have the ability to form hydrogen bonding. These solvents are of two types, acidic or photogenic and basic or protophilic solvents.
- Acidic or protogenic solvents have a strong tendency to donate protons. Examples of such solvents are sulfuric acid, hydrogen fluoride, hydrogen cyanide, etc.
- Basic or protophilic solvents have a strong tendency to accept protons. Ammonia, amines, and hydrazine belong to such classes.
Aprotic Solvent
The solvent that neither donates nor accepts protons is called an aprotic solvent. They may or may not have hydrogen atoms in their formula. Benzene, chloroform, sulfur dioxide, and carbon tetrachloride are examples of aprotic solvents.
Amphoteric Solvent
An amphoteric solvent contains hydrogen in its formula and it donates or accepts protons depending on the nature of the reacting species. It acts as an acid and base. Therefore, it is amphoteric in nature.
These types of solvents dissociate freely into protons and anions. Common examples of amphoteric solvents are acetic acid and water.
Properties
The general properties of solvents depend mainly on
- Dipole moment
- Dielectric constant
- Electrical conductance
- Viscosity
- Proton affinity
Dipole Moment
A solvent having a higher value of dipole moment dissolves polar substances more readily. The greater polarity of a solvent molecule releases greater solvation energy on the dissociation of a solute.
Dielectric Constant
The dielectric constant provides a rough measurement of solvent polarity. The strong polarity of water may be indicated by its high dielectric constant of 88.
Electrical Conductance
Electrical conductance gives an idea about the self-ionizing properties of a solvent.
Viscosity
Viscosity is an important property of a liquid solvent. Some solvents like water, low molecular weight alcohols, and liquid ammonia are highly fluid while anhydrous sulfuric acid and high molecular weight alcohols are highly viscous. Liquid with low viscosity can handle more easily than high viscous liquid.
Proton Affinity
It is applicable for a protonic solvent only. It greatly affects the behavior of a solute in a given solvent system. Ammonia has a greater proton affinity than water molecules. Acetamide behaves as a very weak base in an aqueous solution but it shows acid properties in liquid ammonia.
Examples of Solvent
Some common examples of nonpolar, polar protic, and polar aprotic solvents with their properties are listed below in the table,
Examples of Nonpolar Solvents
| Solvent | Chemical formula | Boiling point (°C) | Density (g/mL) | Dielectric constant |
Dipole moment (D) |
| Pentane | CH3CH2CH2CH2CH3 | 36.1 | 0.626 | 1.84 | 0.00 |
| Hexane | CH3CH2CH2CH2CH2CH3 | 69 | 0.655 | 1.88 | 0.00 |
| Benzene | C6H6 | 80.1 | 0.879 | 2.30 | 0.00 |
| Toluene | C6H5-CH3 | 111 | 0.867 | 2.38 | 0.36 |
| 1,4-Dioxane | C4H8O2 | 101.1 | 1.033 | 2.30 | 0.45 |
| Diethyl ether | CH3CH2-O-CH2CH3 | 34.6 | 0.713 | 4.30 | 1.15 |
| Tetrahydrofuran | C4H8O | 66 | 0.886 | 7.5 | 1.75 |
| Chloroform | CHCl3 | 61.2 | 1.498 | 4.81 | 1.04 |
Examples of Polar Protic Solvents
| Solvent | Chemical formula | Boiling point (°C) | Density (g/mL) | Dielectric constant |
Dipole moment (D) |
| Ammonia | NH3 | -33.3 | 0.674 | 17 | 1.42 |
| Formic acid | H-C(=O)OH | 100.8 | 1.21 | 58 | 1.41 |
| n-Butanol | CH3CH2CH2CH2OH | 117.7 | 0.810 | 18 | 1.63 |
| Isopropyl alcohol (IPA) | CH3-CH(-OH)-CH3 | 97 | 0.803 | 18 | 1.68 |
| n-Propanol | CH3CH2CH2OH | 97 | 0.803 | 20 | 1.68 |
| Ethanol | CH3CH2OH | 78.2 | 0.789 | 24.55 | 1.69 |
| Methanol | CH3OH | 64.7 | 0.791 | 33 | 1.70 |
| Acetic acid | CH3COOH | 118 | 1.049 | 6.2 | 1.74 |
| Water | H2O | 100 | 1.00 | 80 | 1.85 |
Examples of Polar Aprotic Solvents
| Solvent | Chemical formula | Boiling point (°C) | Density (g/mL) | Dielectric constant |
Dipole moment (D) |
| Dichloromethane (DCM) | CH2Cl2 | 39.6 | 1.3266 | 9.1 | 1.60 |
| Ethyl acetate | CH3-C(=O)-O-CH2-CH3 | 77.1 | 0.894 | 6.02 | 1.78 |
| Acetone | CH3COCH3 | 56.1 | 0.786 | 21 | 2.88 |
| Dimethylformamide (DMF) | H-C(=O)N(CH3)2 | 153 | 0.944 | 38 | 3.82 |
| Acetonitrile | CH3-C≡N | 82 | 0.786 | 37.5 | 3.92 |
| Dimethyl sulfoxide (DMSO) | CH3-S(=O)-CH3 | 189 | 1.092 | 46.7 | 3.96 |
| Nitromethane | CH3-NO2 | 100 | 1.1371 | 35.87 | 3.56 |
| Propylene carbonate | C4H6O3 | 240 | 1.205 | 64.0 | 4.9 |
Uses of Solvent
A solvent is a chemical that uses,
- For dissolving paint, oil, and gases.
- Mix or thin pigments, pesticides, glues, epoxy resins, and paints.
- To clean automotive parts, tools, and electronics.
- To make other chemicals.
Therefore, solvents is broadly defined as a liquid or gas which uses for dissolving or extracting other substances in chemical industries.