Surface Tension of Liquid
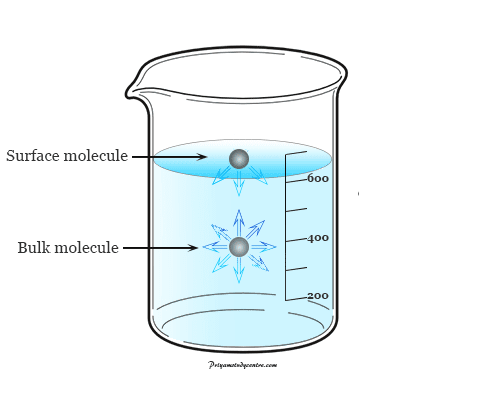
Surface tension or surface energy is the most important characteristic property of liquid origin at the surfaces and is displayed when a liquid contact with its vapour or is stretched the liquid by an elastic membrane. In chemistry or physics, surface tension simply defines the amount of energy that causes to increase in the unit area of the water or any other liquid ( wetting or non-wetting). It is measured by the units dyne cm−1 or Newton meter−1. Consider a molecule in the bulk of the liquid, the molecules are uniformly surrounded by other molecules and attached from all directions. Therefore, the resultant force on the bulk molecule becomes nil.
Origin of Surface Tension
The interfacial molecule is partially surrounded by other molecules and experiences a resultant inward pull. As a result, the molecules on the surface try to leave and enter into the bulk of the liquid or the liquid under tension and try to get the minimum area. This unbalanced force of attraction on these molecules in the liquid is the origin of the properties of surface tension.
Gas to Liquid Conversion
In general, liquids are obtained by cooling gas molecules below their critical temperature with high pressure or from the solid by the specific heat to overcome translational kinetic energy.
The effect of cooling or gas to liquid phase causes to decrease in the thermal energy and high pressure causes to increase in the density, thereby increasing the forces of attraction amongst the molecules.
In the liquid phase, the surface is some sort of tension and tends to contract the smallest possible area in order to contain the minimum number of molecules on the outside. The phenomenon of surface tension in science clearly observed in the spherical shape of small liquid drops (water or raindrops) or soap bubbles in the air.
Surface Tension of Water Bubble
The formation of a bubble is basically due to surface tension or energy. The total pressure acting on the concave side must be larger than the pressure acting on the convex side. Therefore, the pressure inside the bubbles must be larger than the external pressure.
If the external pressure is not balanced by these two forces, then the bubbles are not stable. It immediately collapsed. For getting stable bubbles, these external forces are balanced by other forces namely cementing forces.
Why falling drop of water is spherical?
Water drops in nature displayed a spherical shape with minimum surface area. Due to surface tension, the maximum number of water molecules going on the bulk rather than the surface. For this reason, when water droplets fall freely it takes a spherical shape.
Unit of Surface Tension
It is defined as the force acting along the surface of a liquid at the right angle to any line of unit length. This force is called the cohesive force of the liquid. Numerically and dimensionally, surface tension is equal to surface energy.
It is expressed by force per length having the unit dyne cm−1 in the CGS system and Newton meter−1 in the SI system. The higher the inter-molecular attraction force (cohesive force) of liquid greater will be the magnitude of γ.
Wetting and Non-Wetting Liquids
In learning chemistry, liquids are classified into two types, wetting, and non-wetting liquids, depending on the ability to wet the solid surface or interfacial tension.
- Examples of wetting liquids are water on the glass, alcohol on the glass or cement, and oil on the cloth will easily wet the surface and speed over them.
- Examples of non-wetting liquids like mercury on glass and water on solid paraffin will not be speared or wet.
Contact Angle and Wetting Properties
The properties of wetting and non-wetting depend on the angle of contact on the solid surface. For example, water on glass (angle of contact = 18°) and chemical elements like mercury on the glass (angle of contact = 140°).
From common experience, some liquids spontaneously wet the surface of some solids and speed over them while other liquids do not wet or spread on certain surfaces.
Surface Energy
If the area of the liquid increases, more molecules from the bulk of a liquid to its surface. Some energy is required because work has to be done in bringing the molecules from the bulk against inward attractive forces. The work done in the increasing area by unity is known as the surface energy. The larger the forces of attraction amongst the molecules, the larger will be the net inward pull. It increases net work done for increasing surface area.
With the increasing density of liquids, surface energy decreases. For example, the values of γ for water, benzene, and methanol at 20°C = 72.8, 28.87, and 22.55 dyne cm−1 respectively. The density order for these liquids is,
H2O < C6H6 < CH3OH
Surface Tension and Surface Energy Relation
For the measurement and explanation of surface tension or energy of the liquid, we use a film containing a rectangular ware frame and a moveable membrane. Suppose the film is stretched by moving membrane x meter and length of membrane = l meter.
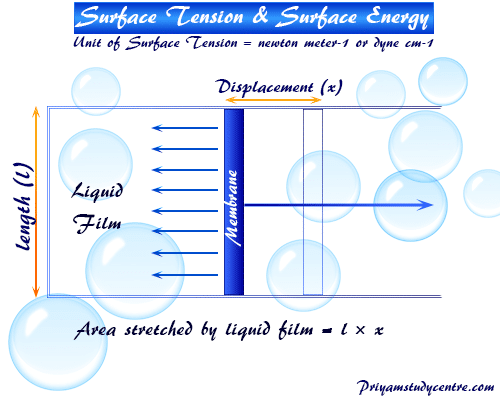
If the γ symbol represented the surface tension acting per meter,
∴ The forces acting towards the film = γ × 2l
Since the length of the film is in contact with the ware l on each side.
Therefore, the total length = 2l
The work required to increase the surface area of the liquids (w),
w = opposing force × displacement
= (γ × 2l) × x
= γ × 2(l × x)
∴w = γ × ΔA
Where ΔA = increase of the area of the film on both sides. Therefore, this mathematical formula uses for the measurement or calculation of the surface energy or tension of any liquid or water.
Unit of Surface Energy
From the above derivation,
surface tension (γ) = w/ΔA or the energy per unit area
It is numerically equal to the surface energy of the pure liquid. The CGS and SI unit of surface energy = Joule meter−2 and erg cm−2 respectively.
Dimension of Surface Energy
According to the formula,
γ = w/ΔA
Therefore, surface tension is the amount of work required to increase the surface area of the liquids by unity. w/ΔA is called surface energy per unit area. It is numerically or dimensionally equal to γ.
The units of these two quantities are different. Unit of surface energy per unit area = erg/cm2 or joule/m2 while the surface tension dyne/cm or newton/m.
The dimensions of surface energy and surface tension are equal to [M T−2]. Therefore, different types of instruments or apparatus like the capillary tube and stalagmometer are used for measuring it.
Effect of Temperature on Surface Tension
It originates from the intermolecular attraction in the liquid. With the rise in temperature, the force of intermolecular attraction decreases. Therefore, the surface tension of the liquid decreases, and at the critical temperature, it will vanish.