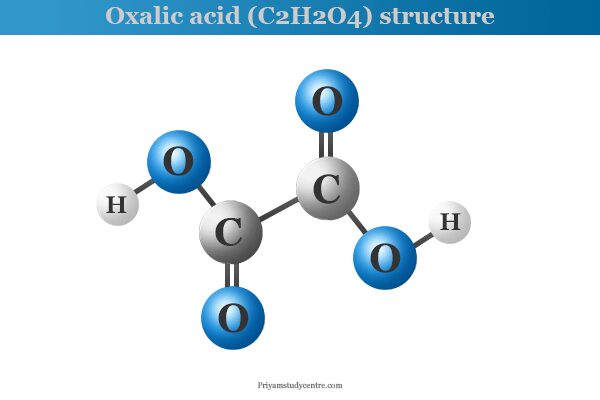
Oxalic Acid Chemical Formula
Oxalic acid or ethanedioic acid (chemical formula C2H2O4) is a poisonous, crystalline, dicarboxylic acid that is present in rhubarb, sorrel, and other plants of the oxalis group. It occurs as a dihydrate with the molecular formula C2H2O4, 2H2O. It is used widely as a cleaning or bleaching agent for the removal of rust or stains. Oxalic acid crystallizes from water as a colourless crystal dihydrate with two molecules of water. The melting point of dihydrate is 101.5 °C but the meting point of anhydrous oxalic acid is 189.5 °C. It is soluble in water and ethanol but almost insoluble in ether. Oxidation of many organic compounds like sugar and starch with nitric acid is used for the production of oxalic acid.
Oxalic Acid Uses
- Oxalic acid is an important household chemical that can be used mainly for cleaning or bleaching and removal of rust.
- It is also used for the manufacture of ink, polishing metal, and removing stains from metal surfaces and clothes.
- In textile industries, the antimony salt of oxalic acid is used as a mordant in printing and dyeing.
- For developing photographic film, we also used it as a reducing agent.
- It is used in the electronic and semiconductor industries for the semiconductor device fabrication process.
The most common individual uses of oxalic acid are given below,
Bleaching
It is a useful bleaching agent for wood and stone. When wood or stone turns gray, we use oxalic acid to bring back its natural colour.
Oxalic Acid for Rust Removal
It is useful for removing rust caused by iron, water, and tannic acid. It is used for cleaning or removing iron rust due to the formation of water-soluble iron salt with ethanedioic acid.
Removing Stains
It is very effective for removing stains caused by ink, and different types of food. Ethanedioic acid removes the stain gently but does not affect the base surface. It is also used for removing strain from linen or cotton clothes.
Production of Oxalic Acid
Industrial Production
- It is prepared industrially by heating sodium formate rapidly to 360 °C.
2 HCOONa → (COONa)2 + H2 - The free acid from its sodium salt can be obtained by adding a calcium hydroxide solution.
- Then calcium oxalate can be treated with the calculated amount of sulfuric acid to form calcium sulfate and C2H2O4.
- Removing calcium sulfate from the solution and evaporating the filtrate to crystallization.
Production in Laboratory
In the laboratory, ethanedioic acid can be prepared by oxidizing sucrose or molasses with concentrated nitric acid in presence of vanadium pentoxide as a chemical catalyst.
C12H22O11 + 18 [O] → 6 (COOH)2 + 5 H2O
Chemical Properties
Oxalic acid is a poisonous acid that crystallizes from water to form colorless dihydrated crystals with the molecular formula C2H2O4, 2H2O. The hydrate loses water when heated at 100 to 105 °C.
When heated above 200 °C, it decomposes to form carbon dioxide, carbon monoxide, formic acid, and water.
| Oxalic acid | ||
| IUPAC name | Ethanedioic acid | |
| Other names | Wood bleach Crab acid |
|
| Chemical formula | C2H2O4 | |
| Molar mass | Anhydrous | Dihydrate |
| 90.034 g mol−1 | 126.065 g·mol−1 | |
| Appearance | white crystals | |
| Odor | odorless | |
| Density | Anhydrous | Dihydrate |
| 1.90 g cm−3 | 1.653 g cm−3 | |
| Melting point | 189 to 191 °C | 101.5 °C |
| Solubility | Soluble in water, ethanol, diethyl ether, etc | |
| Acidity (pka) | 1.25, 4.14 | |
| Vapor pressure | <0.001 mmHg at 20 °C | |
| Conjugate base | hydrogenoxalate | |
| Heat capacity | 91.0 J mol−1 K−1 | |
| Std molar entropy |
109.8 J mol−1 K−1 | |
| CAS Number | Anhydrous | Dihydrate |
| 144-62-7 | 6153-56-6 | |
Chemical Reaction
The anhydrous ethanedioic acid is easily obtained by heating hydrate with carbon tetrachloride.
When heated with concentrated sulfuric acid, it decomposed to form carbon dioxide, carbon monoxide, and water.
(COOH)2 → CO + CO2 + H2O
It is readily oxidized by potassium permanganate (KMnO4) to form carbon dioxide but very slowly oxidized by concentrated nitric acid.
(COOH)2 + [O] → 2 CO2 + H2O
Anhydrous oxalic acid reflux with ethanol to form di-ester like ethyl oxalate.
(COOH)2 + 2 C2H5OH → (COOC2H5)2 + 2 H2O
The more general method for preparing di esters, to heating dicarboxylic acid with alcohol, toluene, and concentrated sulfuric acid.
It is heated with ethylene glycol to form cyclic ethylene oxalate. This reaction is used to differentiate oxalic acid from other dicarboxylic acids. The other dicarboxylic acid reacts with glycol to form polyesters.
The reduction of acid by zinc and sulfuric acid formed glycolic acid (HOCH2COOH). Electrolytic reduction by lead electrode gives glycolic and glyoxalic acid.
For better production of glyoxalic acid, we used reagents like magnesium and sulfuric acid.
Toxicity of Oxalic Acid
The pure concentrated form of oxalic acid is very toxic or dangerous. It is corrosive and harmful if inhaled. It affects mostly the mucous membranes and upper respiratory system of our body.
The acid causes eye irritation and burns our skin. Therefore, we always used latex gloves, protective glasses, and dust masks to prevent the toxic effect of oxalic acid or ethanedioic acid.