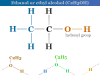
Essential Amino Acids Definition
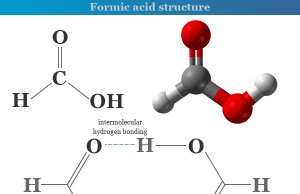
Amino acids are types of organic compounds that contain amino and carboxyl groups in their side chain and are essential for the growth of young animals. Carbon, hydrogen, oxygen, and nitrogen are the key elements found in the amino acid structure. Other chemical elements are also found in the side chain of certain amino acids. When proteins are hydrolyzed by acids, alkalis, or enzymes, they yield different types of amino acids. All the acids obtained from protein are α-amino acid but proline and hydroxyproline are imino-acids. Nine amino acids are essential for the growth of our body. The deficiency in anyone prevents the growth of young animals and may even cause death.
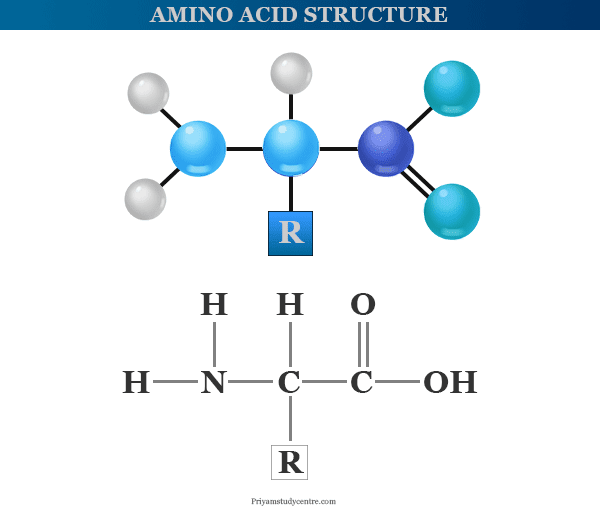
Amino Acids Structure
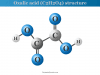
Amino acid molecules are the basic building blocks of proteins that contain amino (−NH2) and carboxyl (−COOH) groups in their structure. Each amino acid has a definite structure. According to the attachment of the amino and carboxyl groups, these are classified as α, β, and γ amino acids.
The names, structures, formulas, and examples of some common amino acids are given below the picture,
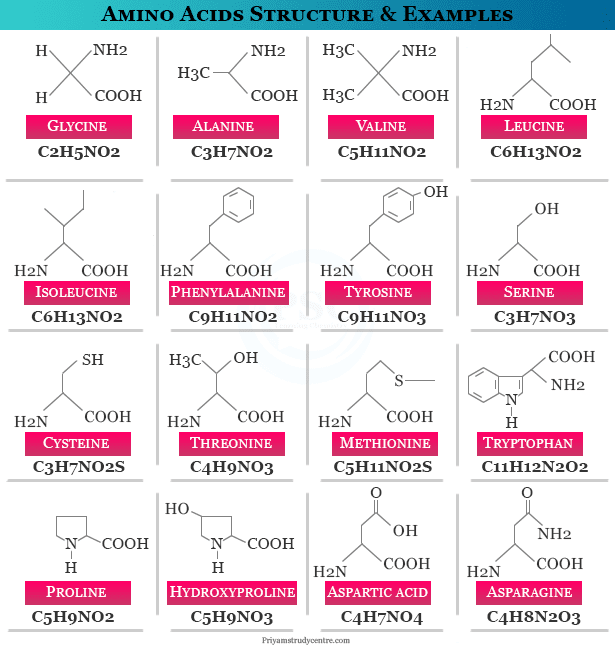
Types of Amino Acid
They can be classified in several ways. They are described by the latter g, l, and e followed by the name of amino acid. The latter g, l, and e indicate the general occurrence, lesser occurrence, and essential.
The 20 amino acids are required for the synthesis of various proteins along with various biological functions. However, all these 20 amino acids need not be taken from our daily diet. Therefore, based on the nutritional requirements, they are grouped into three classes, essential, non-essential, and conditionally essential.
Nonessential Amino Acids
Nonessential amino acids are those acids that are required for normal health and growth. Therefore, they are normally synthesized in our body and cannot be essential to eat extra food to acquire them.
Our body can synthesize 10 amino acids to meet our biological needs. Therefore they need not be consumed in the diet. These are glycine, alanine, serine, cysteine, aspartate, asparagine, glutamate, glutamine, tyrosine, and proline.
Essential Amino Acids
Essential or indispensable amino acids are obtained from foods which we eat because they cannot be synthesized in our body. There are nine amino acids − histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine are essential for the growth of our body.
A deficiency in anyone prevents the growth of our body and may even cause death. Therefore, it is essential to eat food that contains these acids.
Sources of Essential Amino Acids
Animal-based foods such as meat, milk, fish, and eggs are the main sources of essential amino acid. They are also obtained from plant-based food like soy, beans, nuts, and grains.
Conditional Amino Acids
Conditional amino acids are normally not essential but are important in times of illness, injury, or stress. Arginine, cysteine, glutamine, tyrosine, glycine, ornithine, proline, and serine are examples of conditional amino acid.
Classification
Based on the number of amino groups and carboxyl groups, these are classified as neutral, acidic, and basic amino acids.
Neutral
A neutral amino acid contains one amino group and one carboxyl group in its side chain. The systematic name and chemical formula of some common neutral amino acids are given below the table,
| Examples of neutral amino acids | ||
| Name | Systematic name | Formula |
| Glycine | Aminoacetic acid | C2H5NO2 |
| Alanine | α-aminopropanoic acid | C3H7NO2 |
| Valine | α-aminoisovaleric acid | C5H11NO2 |
| Leucine | α-aminoisoacproic acid | C6H13NO2 |
| isoleucine | α-amino-β-methyl-n-valeric acid | C6H13NO2 |
| Norleucine | α-amino-n-caproic acid | C6H13NO2 |
| Phenylalanine | α-amino-β-phenyl-propanoic acid | C9H11NO2 |
| Tyrosine | α-amino-β-(p-hydroxyphenyl)-propanoic acid | C9H11NO3 |
| Serine | α-amino-β-hydoxypropanoic acid | C3H7NO3 |
| Cysteine | α-amino-β-mercaptopropanoic acid | C3H7NO2S |
| Cystine | Bis(α-aminopropanoic acid)-β-disulfide | C6H12N2O4S2 |
| Threonine | α-amino-β-hydroxy-n-butyric acid | C4H9NO3 |
| Methionine | α-amino-γ-methylthio-n-butyric acid | C5H11NO2S |
| Iodogorgic acid | 3,5-di-iodotyrosine | C9H9I2NO3 |
| Thyroxine | β-3,5-di-iodo-4-(3,5-di-iodo-4-hydoxy)-phynyl-α-aminopropanoc acid | C15H11I4NO4 |
| Tryptophan | α-amino-β-indolepropanoic acid | C11H12N2O2 |
| Proline | pyrrolidine-α-carboxylic acid | C5H9NO2 |
| Hydroxyproline | γ-hydoxypyrrolidine-α-carboxylic acid | C5H9NO3 |
Acidic
An acidic amino acid contains one amino group and two carboxyl groups in its side chain. The systematic names and chemical formulas of some common acidic amino acids are given below the table,
| Examples of acidic amino acids | ||
| Name | Systematic name | Formula |
| Aspartic acid | α-aminosuccinic acid | C4H7NO4 |
| Asparagine | α-aminosuccinamic acid | C4H8N2O3 |
| Glutamic acid | α-aminoglutaric acid | C5H9NO4 |
| β-hydoxyglutamic acid | α-β-hydroxyglutaric acid | C5H9NO4 |
| Glutamine | α-aminoglutaramic acid | C5H10N2O3 |
Basic
A basic amino acid contains two amino groups and one carboxyl group in its structure. The systematic name and chemical formula of some common basic amino acids are given below the table,
| Examples of basic amino acids | ||
| Name | Systematic name | Formula |
| Ornithine | α, γ-diamino-n-valeric acid | C5H12N2O2 |
| Arginine | α-amino-δ-guanidino-n-valeric acid | C6H14N4O2 |
| Lysine | α, ε-aminocaproic acid | C6H14N2O2 |
| Histidine | α-amino-β-imidazolepropanoic acid | C6H9N3O2 |
Properties of Amino Acid
In a neutral solution, they exist as dipolar ions. For each amino acid, there is a particular pH scale value at which the concentration of dipolar ion is maximum.
In acid solution, the conjugate acid is predominating but in basic solution, the conjugate is predominating. The dipolar ion is electrically neutral because the net charge is zero. In this condition, they do not migrate when placed in an electric field. The pH at which migration does not occur is called the isoelectric point of amino acid.
Some common properties of amino acid are given below,
- They are colourless crystalline solids.
- They are generally soluble in water but sparingly soluble in organic solvents.
- Most of the acids are melted with decomposition but sublimation is also possible with a number of acids.
- They also contain one chiral center.
- Except for glycine, all the acids are optically active.
- They have the properties to form zwitterion due to the presence of amino and carboxyl groups in their side chain.
- They are amphoteric in nature due to the presence of acidic and basic groups in their side chain.
- They have properties to form peptide linkage.
Ninhydrin Reaction
Ninhydrin (indane 1, 2, 3-trione hydrate) reacts with an amino acid to form a coloured product. Therefore, it is used as a sparing reagent in the identification and qualitative estimation.
- Most of the acids react with ninhydrin to give blue-coloured products.
- However, proline and hydroxyproline give yellow-coloured products.
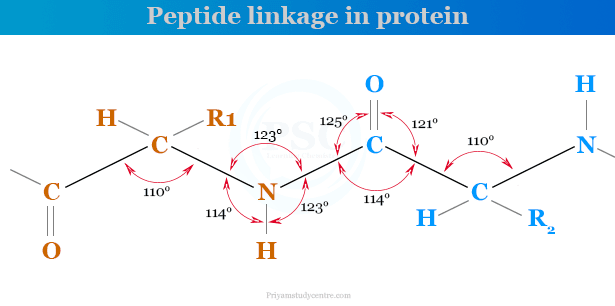
Peptide Linkage in Proteins
Proteins are hydrolyzed by acids, alkalis, or enzymes to form a mixture of amino acids. Therefore, amino acid in proteins is joined linearly by peptide linkages. The atoms in the group of -CONH- are planners. The peptide linkages of amino acids in a protein molecule are given below the picture,