Human Hemoglobin
Hemoglobin or Hb is the iron-containing metalloprotein present in the red blood cells (RBCs) that is responsible for transporting oxygen in the human body through blood. If there is a deficiency or low levels of hemoglobin due to the destruction of RBCs (hemolysis) or loss of RBCs through bleeding or the bone marrow is not able to produce new RBCs fast enough then the overall hemoglobin range will drop down. The structure of the heme group in hemoglobin contains an octahedral complex of iron (II). The center of the octahedron is occupied by the Fe (II) atom and the four corners of the square base contain four nitrogen atoms of the heme group. The fifth position is bound to a nitrogen atom of the imidazole group in the histidine residue while the sixth position is empty.
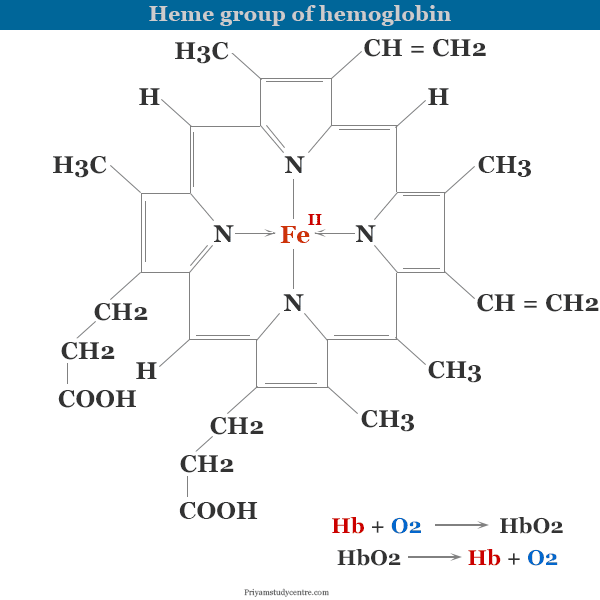
The sixth position of hemoglobin structure is used for transporting oxygen from the lungs to body cells for human beings or animals. Low and high levels of hemoglobin cause different types of health diseases. It causes diseases like anemia and leukemia.
Structure of Heme
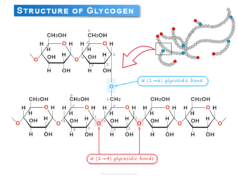
The entire heme structure is encapsulated in a water-repelling pocket of protein chains. In adults, hemoglobin has four protein chains each of which has a heme pocket. The packed form roughly contained a tetrahedral cluster like α2β2. The alpha chains have 141 amino acids residues and the beta chains have 146 amino acid residues.
For infants, the hemoglobin molecule is made up of 2 α chains and 2 γ chains. However, the gamma chains of hemoglobin are gradually replaced by β chains as the infant becomes an adult.
Hemoglobin Normal Range
An adult human contains about 5 liters of blood. Each milliliter of blood contains 5000 million blood cells. Each cell contains 0.25 million Hb molecules. Red blood cells (RBCs) have a relatively short life span of about 100 to 120 days. Therefore, about one percent of Hb molecules in blood are replaced daily.
Hemoglobin normal ranges vary from male to female and newborn to infant. The normal results for hemoglobin ranges are given below the chart,
| Hemoglobin normal range | ||
| For adults | Male | Female |
| 13.8 to 17.2 g/dL | 12.1 to 15.1 g/dL | |
| For children | Newborn | Infant |
| 14 to 24 g/dL | 9.5 to 13 g/dL | |
Function of Hemoglobin
The sixth position of the hemoglobin structure is empty. Therefore, when a human or an animal inhales oxygen from our environment, the gas combines with the Hb of the lungs to form oxyhemoglobin (HbO2).
Oxyhemoglobin formed in the lungs dissociates into Hb and O2 in the cells of the muscles. Hence oxygen becomes available to the body’s muscles or cells.
In other words, we can say that hemoglobin functions as an oxygen carrier from the lungs to tissues through the blood. To function as an ideal carrier of oxygen, oxyhemoglobin must be able to release the dioxygen at the cell to which oxygen can be delivered.
Deoxyhemoglobin
Generally, hemoglobin can be saturated with oxygen molecules to form oxyhemoglobin and desaturated with oxygen molecules to form deoxyhemoglobin. In the absence of oxygen or deoxyhemoglobin is about 36 to 40 pm out of the plane to the nitrogen atoms in the direction of the histidine group.
The structure of oxyhemoglobin and deoxyhemoglobin are given below the picture,
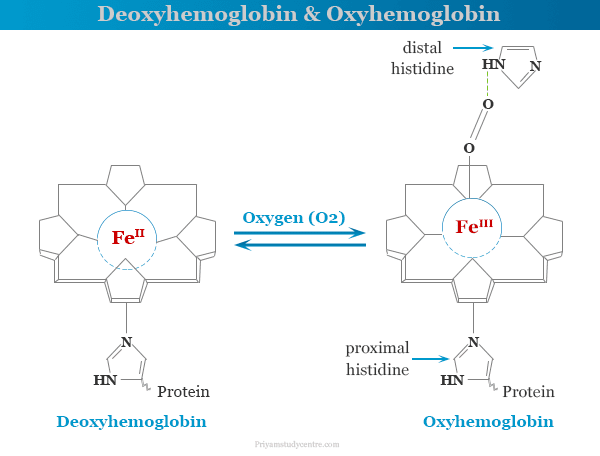
Oxyhemoglobin
Upon binding of dioxygen or oxyhemoglobin, the iron atom moves about 13 pm of the plane and pulls histidine towards it. Therefore, the dioxygen in oxyhemoglobin is bound in a bent fashion. It further makes a hydrogen bond to an N-H chemical bond in an imidazole residue on the side away to form a coordinated imidazole base.
The O-O The stretching band of coordinated O2 molecules lies at 1105 cm−1 which is close to the superoxide ion. Therefore, iron in the oxyhemoglobin complex may be taken as low spin Fe (III).
Deoxyhemoglobin is less efficient in oxygen uptake compared to myoglobin under low oxygen pressure. Here thermodynamically favorable transfer of dioxygen to myoglobin takes place.
The binding of dioxygen by deoxyhemoglobin leads to the release of protons. Therefore, dioxygen release is favored by a decrease in pH scale level. In active muscle, oxygen is converted to carbon dioxide. It releases protons under the action of enzyme carbonic anhydrase which facilitates to release of dioxygen from the oxyhemoglobin complex.
What is Anoxia?
When the oxygen supply to our body cells or brains is completely lost, anoxia happens. It causes suffocation and may even lead to death.
Hemoglobin has a greater affinity for binding carbon monoxide molecules than oxygen molecules. Now if a human being or an animal inhales carbon monoxide instead of oxygen due to air pollution, hemoglobin forms a carboxyhemoglobin complex. The formation of carboxyhemoglobin makes deoxyhemoglobin unable to take up oxygen from the lungs. Therefore, the quantity of oxygen available to the body tissue gets reduced. It is called anoxia.
Hemoglobin Levels
The deficiency of hemoglobin causes diseases like anemia and leukemia. It is identified by symptoms,
- Fatigue
- Feeling triad or cold
- Pale skin
- Headache
- Treble to bathing
If our body does not produce new red blood cells, the hemoglobin level may be dropped down from normal values. Therefore, iron deficiency anemia can be easily treated by eating iron-containing foods or medicine.
Lower Hemoglobin Levels
Lower hemoglobin levels may be developed due to,
- Red blood cells die earlier than the normal life span of 100 to 120 days (hemolytic anemia).
- Hemoglobin levels for women are lowered due to blood loss during monthly periods and childbirth.
- Chronic kidney disease prevents us from making red blood cells.
- When bone marrow does not produce new red blood cells or RBCs, it causes leukemia and other types of cancers.
- Poor nutrition in our diet or eating food that has a low level of iron, folic acid (folate), vitamin B2 (riboflavin), vitamin B12, or vitamin B6.
High Hemoglobin Levels
High hemoglobin levels are caused due to low oxygen levels (hypoxia) in the blood for a long period of time. Common reasons for high hemoglobin levels,
- A rare bone marrow disease that produces too many red blood cells (RBCs).
- Congenital heart disease is present at the time of birth.
- Failure of the right side of the heart.
- Lung diseases like COPD, emphysema, and pulmonary fibrosis.
- Kidney tumors, dehydration, and smoking cause high levels of hemoglobin in the blood.