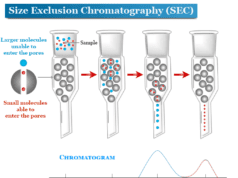
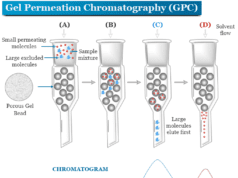
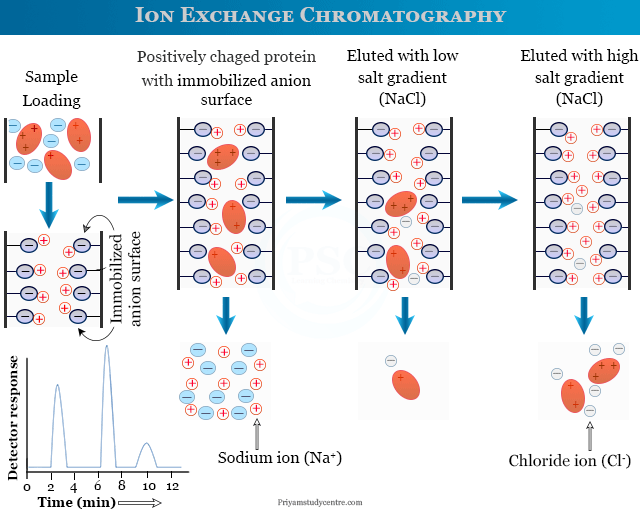
What is ion exchange chromatography?
Ion exchange chromatography or ion chromatography procedure is based on differences in potential between various ions for an ion exchange resin packed in a column. The ion exchange chromatography principle is used mainly for purification of the protein molecule, water softening, and separation of metal. The separation of organic compounds such as amino acids is also done by this procedure.
Cation and anion exchange chromatography are the two types of ion exchange chromatography.
- Cation exchange chromatography is worked for the separation of cations. For example, zirconium, hafnium, niobium, and tantalum have been separated by the cation exchange procedure.
- Anion exchange chromatography is worked for the separation of anions.
Ion exchange chromatography procedure
The ion exchange chromatography procedure is used for the purification of protein in the following way,
- An impure protein sample is loaded on the ion exchange chromatography column given above the picture.
- Positively charged protein molecules are bound to the negatively charged groups of resin molecules.
- A salt gradient is used as an elute to separate protein from resin.
- At a low salt gradient, a few positively charged protein molecule is eluted from the resin column. At a high salt gradient, most of the positively charged protein molecule is eluted from the resin column.
Various factors influence the behavior of elution. The volume of eluent increases when the particle size of the ion exchanger increases.
The pH scale value is used to elute protein on the basis of its isoelectric point. At an isoelectric point, amino acids in the protein molecule do not carry any change and they do not migrate towards an electric field.
- A decreasing pH gradient is used for the elution of protein from anion exchange resin.
- An increasing pH gradient is used for elution protein from cation exchange resin.
Ion exchange chromatography principle
Various explanations are used to explain the ion exchange chromatography principle. Pauling and Bragg draw the similarities between ion exchange chromatography and ionic crystalline solids.
In ionic crystals such as potassium chloride, each potassium ion (K+) is surrounded by eight chloride ions (Cl−). When we placed a medium with a high dielectric constant like water, the net attractive forces between the ions are reduced. Therefore, an exchange of ions in the solution is carried out. If we added NaNO3 in KCl solution, potassium ion exchange with sodium ion and Cl− exchange with NO3− ion.
The mechanism of noncrystalline ion exchange resin is quite similar to that of an ionic crystal. The resins are high molecular weight insoluble polymers or electrolytes. The functional groups such as HSO3H, COOH, and OH are responsible for exchanging ions.
Double layer
Helmholtz’s double layer consists of two rigid electrical layers at the surface of the colloid. It is similar to the plates of a condenser. A double layer contains an inner fixed layer with an outer mobile layer of charges. The concentration of the mobile layer or defuse layer depends on the concentration and pH scale of the external solution.
When we added a foreign ion to the external solution, new ions will enter the outer layer by replacing some ions held on the layer. A new equilibrium is set up and the overall exchange is stoichiometric.
Donnan membrane theory
Donnan membrane theory deals with the unequal distribution of ions on two sides of a membrane. One side contains an electrolyte whose ions cannot penetrate through the membrane.
In ion exchange equilibria, the interface between solid and liquid phases may be considered a membrane.
Ion exchange resin
The name ion exchange resin suggests that constitute contains resinous polymers which are capable of exchanging ions. Depending on the types of ion exchange, there are two types of resin,
- Cation exchange resin
- Anion exchange resin
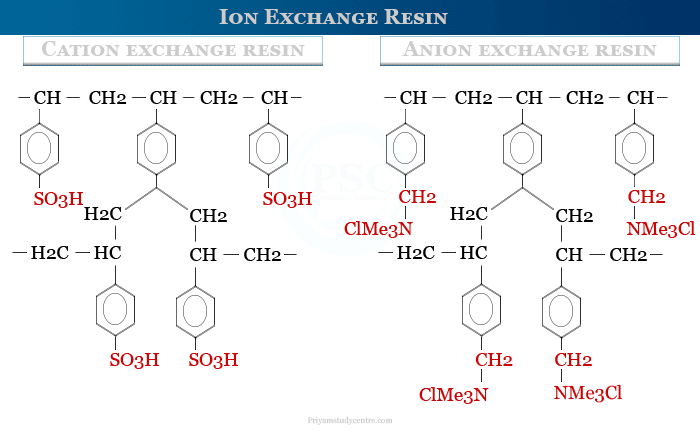
Cation exchange resin
A common synthetic cation exchange resin is obtained by polymerization of styrene and a small amount of divinylbenzene followed by sulfonation. Hydrogen ion (H+) is the exchangeable cation of this resin.
When we treat this resin with Na+ ions, its H+ ions will be replaced by sodium ions. In cation exchange, usually, the cation with high valency is strongly retained but monovalent ions are easily removed.
Anion exchange resin
An anion exchange resin is a polymer having amines or quaternary ammonium groups contained in the polymeric network. In this resin chloride ion is an exchangeable ion and the rest is non-exchangeable. In anion exchange procedure, an anion with a high charge is strongly retained.
Instrumentation of Ion exchange
A schematic diagram of overall ion exchange chromatography instrumentation is given below the picture,
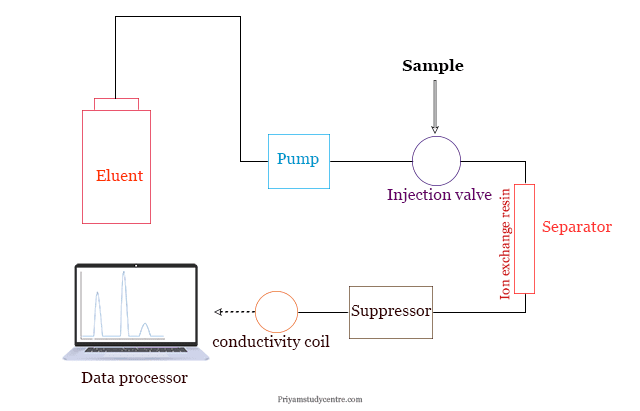
- Inert materials like Kel-F, Teflon, and polypropylene are used for the construction of columns in ion exchange chromatography procedure. The sample is usually added to the top of the column.
- A double piston pump is used which can electronically control the flow of liquids with accuracy.
- The pumps control the rate of flow of the samples to the operator column.
- The column contains ion exchange resin specially manufactured for ion exchange chromatography.
- A microprocessor technology can bosted the use of conductivity detection of ions in nano concentration rages.
- The eluted samples pass through the suppressor column to release the ion or solution with high conductivity.
- The signal is fed to the recorder. The concentration of ions can be measured from the elution profile.
Applications of ion exchange chromatography
Ion exchange chromatography is the most widely used chromatographic method for the purification and separation of cations and anions. It is used mostly for the separation or purification of proteins, amino acids, softening of water, and separation of metal.
Apart from the separation of cations and anions in analytical chemistry and biological chemistry, it is also used in different fields such as medicine, agriculture, pharmaceutical, and petrochemical industries.
- In medicine, it is used for the analysis of sugar and amino acids. It is also used for the separation and purification of blood components.
- It is used for the analysis of impurities or quality control in chemical industries.
- In agriculture, it is used for the analysis of micronutrients in soil samples.
- In the petrochemical industry, it is used for the determination of sulfur compounds.
- In environmental chemistry, the ion exchange chromatography procedure is used for the detection and separation of toxic pollutants present in an aquatic and atmospheric environment.