What are Radioactive Isotopes?
Radioactive isotopes or radioisotopes of a radioactive element are atoms of the same element with different mass numbers. Isotopes of a particular radioactive element have the same number of protons (atomic number) but varying numbers of neutrons inside the nuclei. Isotopes possess identical chemical properties due to their identical electronic configuration. It is formed due to the spontaneous radioactive decay of alpha, beta, and gamma particles in the form of energy. Radioactive isotopes of lead, uranium, thorium, iodine, sodium, and cobalt are used by researchers in radioactive medicine in radiotherapy, biology, agricultural chemistry, trace chemical analysis, and radioactive nuclear power generation process. The radioactive decay series shows different isotopes of radioactive elements given in the picture,
Radioactive Isotopes Examples
Chlorine-35 and chlorine-37, carbon-12 and carbon-14, oxygen-16 and oxygen-18, hydrogen-1 and hydrogen-2 are the most common examples of isotopes.
Continuous emission of alpha, beta, and gamma rays from radioactive uranium and thorium formed the most stable non-radioactive isotopes of lead. This process generally formed the list of radioactive decay series or chain and radioactive isotopes in nuclear physics and chemistry.
Alpha and Beta Radiation
Alpha Decay Example
The decay of alpha rays within the radioactive atoms loses two units of atomic number and four units of mass number. For example, if a radioactive isotope has a mass number = M and atomic number = Z. Therefore, after the emission of the alpha particle, the atomic and mass number of the newborn radioactive isotope = (Z − 2) and (M − 4) respectively.
Emission of Neta Particle
The emission of a beta particle from the nuclei of the radioactive isotope increases the atomic number by one unit and mass numbers remain unchanged. For example, when an element ejected a beta particle the atomic number of the newborn radioisotope = (Z + 1).
Group Displacement Law
In 1913 Soddy define the law of radioactivity to describe the position of the radioactive isotopes or radioisotopes in the periodic table known as the group displacement law. This law states that when an alpha particle is emitted in a radioactive decay step, the product is displaced two places to the left in the periodic table but the radiation of beta particles results in a displacement of the product to one place to the right.
In the year when Soddy proposes the group displacement law, the knowledge of the atomic structure or electronic orbital configuration was still incomplete. He proposes this law through extensive chemical studies on the disintegration products of different radioisotopes. Soddy coined the term isotopes for such elements as they occupy the same position in the periodic table.
Radioactive Decay Series
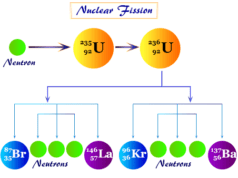
Uranium is the first discovered radioactive f-block elements with an atomic number of 92. Since uranium-238 successive decay to form the most stable isotope of lead-208. It is an example of a radioactive decay series.
The radioactive elements continue to successive disintegration till the daughter elements become a stable non-radioactive isotope of lead. The mother elements along with all the daughter elements are called the radioactive decay series.
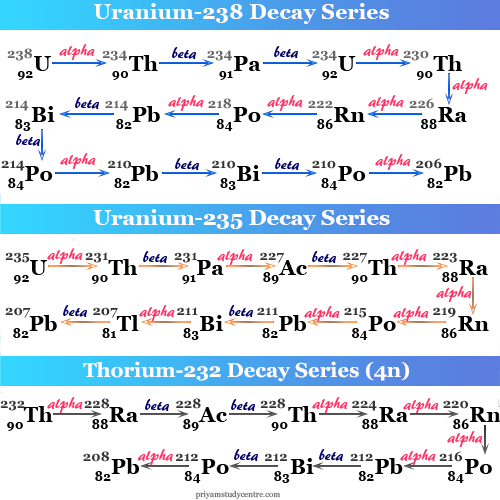
Uranium-238 Decay Series
Unstable uranium-238 decays to form the most stable radioactive isotope of lead-206. The entire route involves eight alpha and six beta emissions.
92U238 → 82Pb206 + 8α + 6β
The mass number of all the decay products is given by 4n +2 (n = 59 for uranium-238). Therefore, these series are known as the 4n +2 series ( n = an integer).

Uranium-235 Decay Series
Radioactive uranium-235 or (4n+3) series starts with uranium-235 and ends with lead-207. Therefore, seven alpha and four beta radiate in the entire route of radioactive emission.
92U235 → 82Pb207 + 7α + 4β
Thorium-232 Decay Series
Unstable radioactive thorium-232 undergoes successive decay to form the most stable radioactive isotope of lead with mass number 208. Therefore, the entire decay chain of thorium-232 involves six alpha and four beta emissions. This is called the 4n radioactive decay series.
90Th232 → 82Pb208 + 6α + 4β
Uses of Radioactive Isotopes
A list of radioactive isotopes like hydrogen, carbon, oxygen, nitrogen, sodium, cobalt, iodine, and sulfur are used in different disciplines like research, medicine, agriculture, biology, trace analysis, and analytical problems. The uses of radioactive isotopes may be classified into the following types,
- Uses of radioactive isotopes in medicine
- Uses of radioisotopes in chemistry
- Radiocarbon dating
- Uses of radioisotopes in agriculture
Uses of Radioactive Isotopes in Medicine
Radiation therapy of radioactive isotopes uses mostly as a weapon to kill the affected tissue in the human body in our daily life. It is used mainly in radiotherapy for cancer treatment or used as electromagnetic spectrum radiation in medicine.
Iodine-131 Treatment
A radioactive isotope of iodine-131 uses in external radiation therapy for the treatment and diagnosis of thyroid gland disorders in the human body.
During drinking a solution of sodium iodide containing iodine-131, the radioactive iodine moves preferentially to the thyroid gland. The beta emission from iodine-131 destroys the malignant cells without affecting the rest of the body.
Uses of Cobalt-60
The radioactive cobalt-60 isotope is an example of a gamma emitter with definite wavelengths. It is used mostly in radiation therapy to inhibit the malignant tissue or treatment of cancer in radiation therapy.
Uses of Sodium 24
Sodium-24 isotope is used in the abnormality of blood circulation in the human body. Therefore, the doctor injected the vein of the patient with sodium salt containing sodium-24 isotope.
Phosphorus-32 Used in Blood Cancer
Phosphorus-32 isotope is used mostly in leukemia therapy or the treatment of blood cancer. Leukemia is the overproduction of white blood cells in the human body.
Radioactive Isotopes in Chemistry
Radioactive isotopes have played a key role in the evaluation of reaction mechanisms and chemical kinetics in chemistry.
Isotopic Labeling Examples
The mechanism of esterification is easily evaluated by using an oxygen isotope. The process of esterification generally has two possible mechanisms.
- First carboxylic acid OH may react with the hydrogen atom of the alcohol to eliminate water.
- The second alcohol OH reacts with carboxylic hydrogen of acid.
If the alcohol oxygen is labeled with O-18, then two cases arise, the ester will carry O-18 or the ester does not carry O-18 but water carries the O-18 isotope. In reality, both possible mechanisms have been observed.
Isotopic labeling of sulfur-35 has also been used to establish the non-equivalency of the two sulfur atoms in the thiosulfate ion.
All the iodine in the HgI4– ion is equivalent proved by isotopic labeling of iodine.
Uses of Radioisotopes in Agriculture
Radioactive isotopes in agriculture are mostly used to determine essential constituents intake from fertilizer and the same constituents from the soil.
For example, we were given some superphosphate to the plants labeled with phosphorus-32 (a beta emitter). The plants do not distinguish between radioactive phosphorus from superphosphate and phosphorus from soil.
After a certain period of growth, the plants are burnt. The quantity of phosphorus and phosphorus-32 in the ash can be determined during chemical analysis. The fact tells us that the amount of labeled radioactive isotope consumed by the plant.