Nuclear Fusion Energy
Nuclear fusion is a nuclear reaction where very light nuclei of chemical elements can be joined or fused to form heavier elements by the emission of energy or loss of masses. In the nuclear fusion process, if two protons and two neutrons are fused or two deuterons are united, the produced energy is very large about 1.2 MeV. Nuclear fusion technology generally works to overcome the repulsive barrier in the nuclear fission reactor of the nuclear power plant. The nuclear fusion reaction that occurs in the interior of the sun and stars is the source of solar stellar energy.
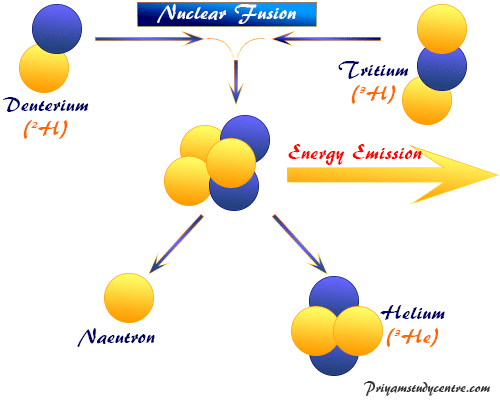
Nuclear Fusion Technology
Fusion technology or formula was first initiated in the Second World War for the preparation of thermonuclear weapons and hydrogen bombs.
Today, fusion technology is used mostly in the nuclear reactor of power plants for electric or nuclear power generation in peaceful applications to mankind.
Nuclear Fusion in the Sun
Nuclear fusion reactions initiated by high temperatures are called thermonuclear reactions.
41H1 → 2He4 + 21e0 + energy (ΔE)
The above net fusion or thermonuclear reaction occurs in the interior of the sun or star of our solar environment. Therefore, the temperature of the sun or the decent star is of the order of twenty million degrees Celsius.
The calculated energy output during the nuclear fusion reaction in the sun loses mass at the rate of four million tons per second. Even with this huge mass loss, our sun will continue to bless us with a good Earth solar environment for thirty billion years.
How does Nuclear Fusion Work?
Thermonuclear bombs like hydrogen bombs work through the nuclear fusion process. The conditions to create hydrogen bombs have been not revealed. The common fusion process in chemistry or physics generally works through joining or fusing neutrons, deuterium, or tritium (atomic number =1 and mass number = 2 or 3) by the liberation of a huge amount of heat or energy.
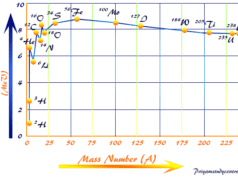
The energy released in the fusion of nuclei originates from the fact that the loss of mass occurs in the artificial nuclear reaction. Therefore, energy release in the fusion process can be calculated from the mass defect and Einstein’s mass-energy equation. It can be used to overcome repulsive barriers in the fission process.
In the bomb, there is a small core of plutonium fission detonator. It is surrounded by a thick cover of fusible material which turns to enclosed by uranium. The fast neutrons produced from the fusion process lead to the fission of surrounding uranium. The fusion process is not controlled by critical size. Therefore, very large hydrogen bombs are produced by this process.
Nuclear Fusion Power Generation
Nuclear fusion energy is the amount of energy released during the fusion reaction. It can be calculated from the mass defect. Let us take the nuclear transformation,
21H1 + 20n1 → 2He4
The mass defect,
= (2 × 1.00814) + (2 × 1.00898) − 4.00388
= 0.3424 amu
Therefore energy released during the fusion process,
= 0.03424 × 931 MeV
≈ 28 MeV
Where 1 amu = 931 MeV given from Einstein equation.
| Nuclear fusion | Fusion energy |
| 21H2 → 2He4 | 24 MeV |
| 1H3 + 1H2 → 2He4 + 0n1 | 17 MeV |
| 1H3 + 1H1 → 2He4 + 0n1 | 20 MeV |
| 1H3 + 1H2 → 2He4 + 20n1 | 11 MeV |
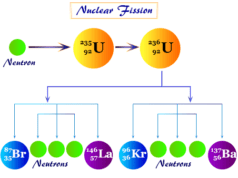
Difference Between Fission and Fusion
During nuclear fission, a natural particle approaches radioactive uranium-235 without any formation of the repulsive barrier. During fusion, positively charged particles have approaches to each other by the formation of the repulsive barrier.
The approaching nuclei must be supplied with sufficient kinetic energy to overcome the repulsive barrier. To overcome the repulsive barrier in the reactor, we mostly use fusion technology in the preliminary step for the initiation of extremely high temperatures or energy.
Stellar Energy
In learning chemistry or physics, stellar energy means the continuous emission of enormous amounts of energy from the sun and other stars due to the fusion process.
The temperature of the interior of the stars is of the order of 107 to 108 K. Such temperature was attained during the construction of the original gaseous nebulae. During such high temperatures, the atoms are completely stripped of their electrons. The bare nuclei with extremely high thermal energy fuse together at a rapid rate which is reasonable for the colossal output of energy.
In 1938, Bethe and Weizsacker independently proposed a theory in which 4 helium nuclei are formed in the stars or sun. During the fusion of four protons, a series of changes occurs in the isotopes of carbon, nitrogen, and oxygen.
The net stellar reaction,
41H1 → 2He4 + 21e0 + energy
Therefore, the nuclear fusion process is the main source of solar stellar energy.