Uranium Metal
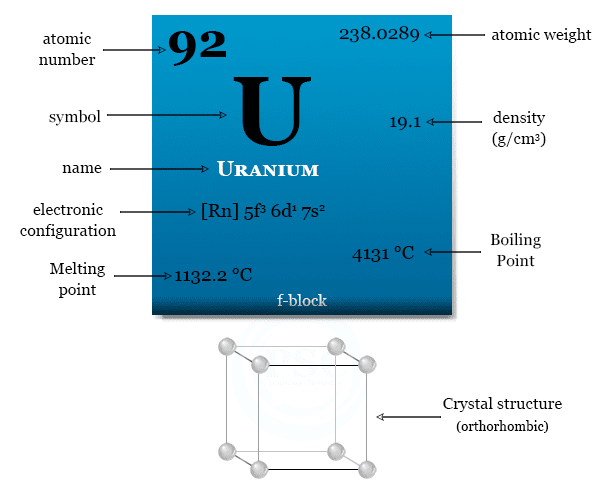
Uranium is a silvery-white radioactive chemical element or metal present in the f-block of the periodic table with the symbol U and atomic number 92. It was discovered in 1789 by Martin Heinrich Klaproth in a specimen of pitchblende. Uranium uses mainly for nuclear energy production in nuclear power reactors. Ordinary uranium contains mostly U-238. It can be enriched in U-235 for the production of nuclear energy. The electronic configuration of uranium is [Rn]5f36d17s2. Therefore, it has six electrons outside the stable radon configuration. Uranium reacts with almost all non-metal elements (except noble gases) and their compounds.
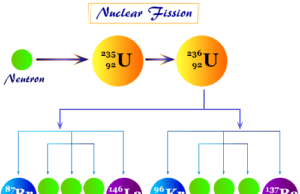
Naturally occurring uranium contains only one percent fissionable U-235 and 99 percent U-238. It can decay slowly by emitting an alpha particle.
- Uranium-235 is the only naturally occurring fissionable fuel that can participate in a chain reaction. It can be used mainly in nuclear power plants and the production of nuclear weapons.
- U-238 is fissionable by fast neutrons. Therefore, it can be transmuted to fissile plutonium-239 by a nuclear reaction.
The half-life of U-238 is about 4.47 billion years and that of U-235 is 704 million years. The metal is very useful for calculating the age of the earth.
Where Uranium is Found?
Naturally occurring uranium contains mainly 238U and 235U. Most of the uranium used in nuclear reactors is found in six countries such as Kazakhstan, Canada, Australia, Namibia, Niger, and Russia.
The most important source of uranium is pitchblende (U3O8). Pitchblende is found mostly in the Great Bear Lake in Canada. A significant amount of uranium ores like Carnotite is found in Siberia in Russia and the United States.
How Uranium is Mined?
Uranium occurs naturally in low concentrations in soil, rock, and water. It is commercially extracted from uranium-containing minerals such as uraninite. Uranium mines in the world go through open pit, underground, in-situ leaching, and borehole mining.
- Low-grade uranium ore mined from various countries contains 0.01 to 0.25% uranium oxides.
- High-grade ores found in Athabasca Basin deposits in Saskatchewan, Canada can contain up to 23% uranium oxides.
Uranium Production
The world production of uranium increased in the present day because it is an important metal for energy generation. Worldwide production of uranium mines in the world in 2013 was obtained from three countries such as Kazakhstan, Canada, and Australia.
Other important uranium mining countries in the world may include,
- Canada (9,331 tonnes)
- Niger (4,518 tonnes)
- Namibia (4,323 tonnes)
- Russia (3,135 tonnes)
Production Process
The production or extraction of uranium from pitchblende contains the following main steps,
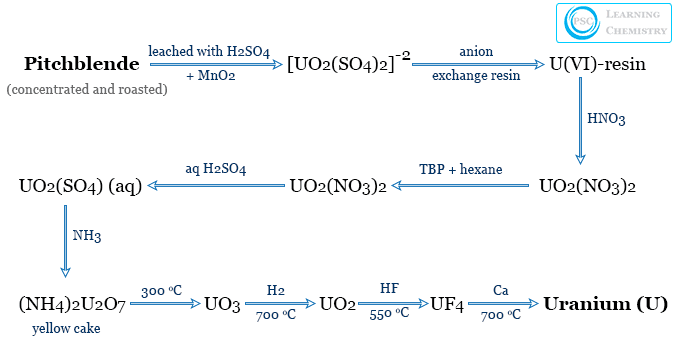
- Pitchblende is concentrated through gravity separation by suspension in water. The concentrated ore is roasted to eliminate moisture, arsenic, antimony, etc.
- The roasted ore is digested with 1:1 sulfuric acid in the presence of MnO2 or NaClO3 to ensure the oxidation of the metal to form U (IV).
- The solution containing U (IV) is boiled with excess sodium carbonate to precipitate sodium diuranate. The diuranate dissolves to form a carbonato complex. Iron, aluminum, and manganese passed into the insoluble precipitate. Therefore, iron, aluminum, and manganese are removed by filtration.
- The filtrate is treated with HCl/H2S to precipitate lead and copper. The filtrate is freed from hydrogen sulfide and treated with ammonia to form a yellow cake or ammonium diuranate.
- Presently disulfatouranylate complex anion is concentrated on an ion exchange resin. It is extracted with concentrated nitric acid. The uranyl nitrate obtained by this step is purified by solvent extraction. It is now precipitated as a yellow cake.
- The yellow cake is now heated at 300 °C to yellow UO3. It is reduced with hydrogen at 700 °C to form black UO2. The dioxide is converted to UF4 by heating with HF at 550 °C.
- Uranium metal is finally obtained by reducing UF4 with calcium or magnesium at 700 °C. The metal is separated from slag and solidified. It is remelted in a vacuum to obtain pure uranium.
Properties
Uranium is a silvery-white malleable, ductile, slightly paramagnetic, radioactive metal that has strongly electropositive properties. Uranium metal is denser than lead but slightly less dense than tungsten and gold.
| Properties of uranium | |
| Name | Uranium |
| Symbol | U |
| Naming | after planet Uranus |
| Discovery | Martin Heinrich Klaproth in 1789 |
| First isolation | Eugène-Melchior Péligot in 1841 |
| Appearance | Silvery-white radioactive element |
| Standard atomic weight | 238.029 |
| Main isotopes | 234U, 235U, 238U |
| Periodic properties | |
| Atomic number | 92 |
| Group | Actinides |
| Period | period 7 |
| Block | f-block |
| Electrons per shell | 2, 8, 18, 32, 21, 9, 2 |
| Electron configuration | [Rn] 5f3 6d1 7s2 |
Uranium in the periodic table
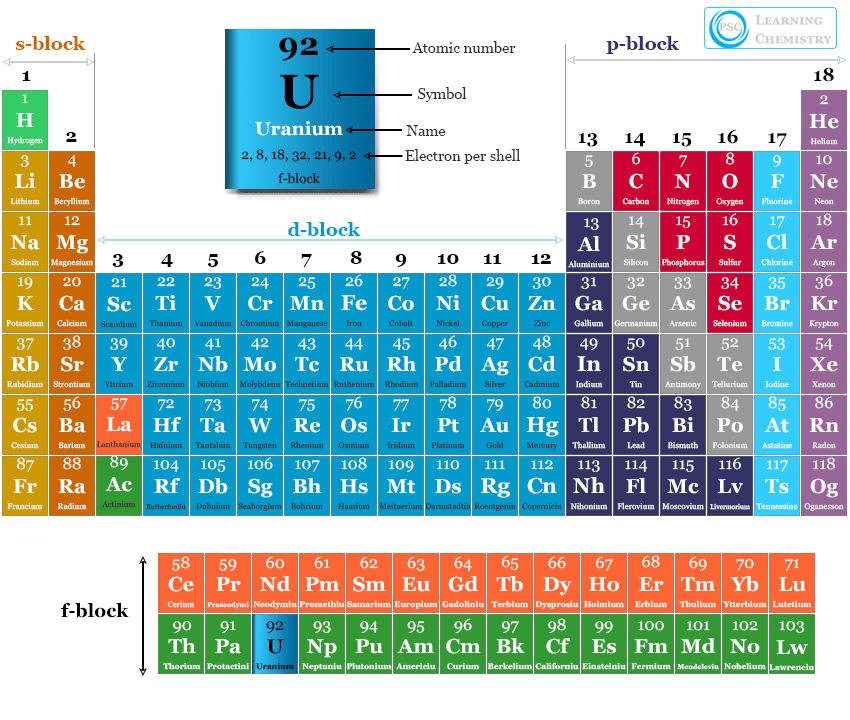
The uranium atom has 92 electrons and 92 protons with electronic configuration [Rn] 5f3 6d1 7s2. It has an incomplete f-orbital. Therefore, uranium is placed in period 7 in the periodic table within the f-block elements or actinides.
Physical Properties
| State at 20°C | Solid |
| Melting point | 1132.2 °C |
| Boiling point | 4131 °C |
| Density | 19.1 g/cm3 |
| Heat of fusion | 9.14 kJ/mol |
| Heat of vaporization | 417.1 kJ/mol |
| Molar heat capacity | 27.665 J mol−1 K−1 |
| Crystal structure | Orthorhombic crystal lattice |
Chemical Properties
| Common oxidation states | +6, +5, +4, +3 |
| Electronegativity | 1.38 (Pauling scale) |
| Ionization energies | 1st ionization energy: 597.6 kJ/mol |
| 2nd ionization energy: 1420 kJ/mol | |
| Atomic radius | 156 pm (empirical) |
| Covalent radius | 196±7 pm |
| Van der Waals radius | 186 pm |
| Magnetic Ordering | paramagnetic |
| Thermal conductivity | 27.5 W m−1 K−1 |
| CAS Number | 7440-61-1 |
Uranium shows mainly four oxidation states III, IV, VI, and VI. The VI state is the most stable oxidation state of the metal.
It reacts with almost all non-metal elements and their compounds. The reactivity of metal increases with the increasing temperature.
It can be dissolved by hydrochloric acid and nitric acid but react slowly with non-oxidizing acid. The finely divided metal reacts with cold water. In the air, it becomes coated with a dark layer of UO2.
Uranium Compounds
Uranium shows mainly four oxidation states III, IV, VI, and VI. Of these four oxidation states, VI is the common and most stable oxidation state. The main uranium compounds formed by these different oxidation states are given below,
Oxides
Uranium formed three main oxide compounds UO3, UO2, and U3O8. Two other less familiar oxides are U3O7 and U4O9.
- The yellow trioxide (UO3) may be obtained by heating uranyl nitrate, hydroxide, or peroxide to 350 °C. It is also produced from ammonium diuranate when heated below 580 °C.
- Black dioxide (UO2) is formed by the reduction of UO3 with hydrogen or carbon monoxide at 350 °C.
- Green black U3O8 is formed by heating UO3 to 700 °C.
All these four oxides dissolved in nitric acid to form a solution containing uranyl ions. The fusion of uranium oxides with alkali and alkaline earth metal carbonates produces uranates and diuranates.
Halides
Uranium forms a number of stable halide compounds in different oxidation states. The halide compounds of uranium are given below in the table,
| Oxidation states | |||
| VI | V | IV | III |
| UF6 colorless |
UF5 pale blue white |
UF4 green |
UF3 green |
| UCl6 green |
U2Cl10 red |
UCl4 green |
UCl3 red |
| UBr4 brown |
UBr3 red |
||
| UI4 black |
UI3 black |
||
Uranyl Nitrate
Uranyl nitrate has the chemical formula UO2(NO3)2. It is obtained when UO3 or any other oxide of uranium metal dissolves in nitric acid.
UO2(NO3)2 is freely soluble in water, alcohol, and ether. The crystalline forms which contain three and two molecules of water may be obtained from the concentrated and fuming nitric acid solution respectively.
Uranyl Sulfate
Uranyl sulfate (chemical formula UO2SO4, 3H2O) may be prepared by evaporating uranyl nitrate with concentrated sulfuric acid followed by leaching with water. Yellowish-green crystals may be isolated from the concentrated solution.
Conductivity measurement shows that the concentrated solution of uranyl sulfate contains the complex ion [UO2(SO4)2]−2.
Sodium Diuranate
A yellow precipitate of sodium diuranate (chemical formula Na2U2O7, 6H2O) is obtained by adding sodium hydroxide solution to a solution of uranyl salt.
2 UO2(NO3)2 + 6 NaOH → Na2U2O7 + 4 NaNO3 + 3 H2O
It is used for painting porcelain and making fluorescent glass.
Uranyl Chloride
Anhydrous uranyl chloride (UO2Cl2) may be prepared by the action of chlorine on UO2 at 500 °C or the action of oxygen on UCl4 at 300 °C. A solution of UO3 in HCl yields yellow-green crystals of UO2Cl2, 3H2O.
Ammonium Diuranate
Ammonium diuranate is obtained by the addition of aqueous ammonia to a solution containing uranyl nitrate
It is soluble in ammonium carbonate to form a carbonate complex. On heating below 580 °C ammonium diuranate decomposes to UO3. U3O8 is formed above 580 °C.
Uranyl Carbonate
Uranyl carbonate (UO2CO3) is present in the mineral rutherfordine. It may be precipitated from solutions containing the uranyl ion with ammonium or sodium carbonate.
Uranyl Acetate
Uranyl acetate has the chemical formula UO2(CH3COO)2, 2H2O. It may be prepared by dissolving UO3 in acetic acid and crystallizing.
Isotopes
Naturally occurring uranium metal contains the three most common isotopes 234U, 235U, and 238U. All these three isotopes are radioactive. They emit alpha particles and they have a small probability of undergoing spontaneous fission reactions. It has also four trace isotopes U-239, U-237, U-236, and U-233.
- Uranium-238 is formed when 238U undergoes spontaneous fission.
- U-237 is formed when 238U captures a neutron.
- Uranium-236 occurs in trace quantities due to neutron capture on 235U.
- U-233 is formed in the decay reaction of neptunium-237.
| Isotope | Abundance | Atomic mass | Half life | Decay Mode | Product |
| 233U | trace | 233.040 | 1.592×105 years | Spontaneous fission (SF) | |
| α | 229Th | ||||
| 234U | 0.05% | 234.041 | 2.455×105 years | Spontaneous fission (SF) | |
| α | 230Th | ||||
| 235U | 0.720% | 235.044 | 7.04×108 years | Spontaneous fission (SF) | |
| α | 231Th | ||||
| 236U | trace | 236.046 | 2.342×107 years | Spontaneous fission (SF) | |
| α | 232Th | ||||
| 238U | 99.274% | 238.051 | 4.468×109 years | α | 234Th |
| Spontaneous fission (SF) | |||||
| β– β– | 238Pu |
What is Uranium Used For?
- Uranium is a very important metal in the periodic table because it is used widely for the generation of electrical energy in nuclear power reactors.
- It is used widely for the production of nuclear weapons. Two major types of fission bombs were produced from U-235 and plutonium-239 obtained from U-238 during the later stages of World War II.
- It is used for the production of military ammunition. This ammunition contains depleted uranium alloyed with 1–2 percent of other metals like titanium or molybdenum.
- Small amounts of uranium metal are used for the production of yellow glass and pottery glazes before the discovery of radioactivity.
Uranium for Nuclear Power
It is the most widely used fuel in nuclear power plants. It is considered a nonrenewable energy source found in rocks. Thermonuclear reactors are based on U-235. Uranium is a more common metal than silver but U-235 is relatively rare. Therefore, ordinary uranium which contains mostly U-238 has to be enriched in U-235 for use as nuclear fuel.
Most of the uranium mined from Kazakhstan, the United States, Canada, Australia, and Russia has to be enriched in U-235 for nuclear fission reaction. It is achieved by a multistage diffusion process and a gas centrifuge process.
Another alternative promising process depends on the appreciable difference in ionization energies of U-235 and U-238. A mixture containing both kinds of atoms in a vapor state is subjected to a laser beam when only the U-235 atoms are ionized. The ions are subsequently collected on a negative electrode. It is called laser isotope separation.
Nearly fifty percent of the total reactor requirement of uranium comes from reprocessing of nuclear fuels. Many of the fission products in a nuclear power reactor strongly absorb neutrons. They have to clear from time to time for the smooth running of the nuclear reactor. These fission products are separated from the unchanged uranium for further use in energy generation.