Facts About Carbon
Carbon is a nonmetallic chemical element of group-14 (IVA) in the periodic table with atomic number 6 and symbol C. The first two elements of the carbon family occupy a special position in our lives and environment. It occurs in nature in various allotropic forms (carbon black, activated carbon, graphite, diamond, etc) and many organic or inorganic compounds (hydrocarbons, carbon dioxide, carbon monoxide, carbonates, etc). Various allotropic forms of carbon have been uses in many production processes. The ratio of carbon isotopes (C-14 and C-12) is also used in radiocarbon dating or determination of the age of plants or animals. Carbon has the facts to form a large number of compounds by bonding with itself and many other chemical elements. Due to such properties, carbon can form millions of organic compounds with hydrogen (hydrocarbons) and many other metals and nonmetals.
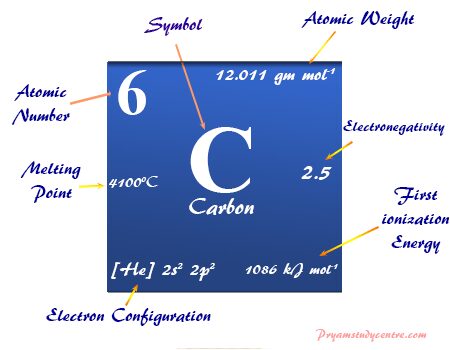
The electronic configuration of carbon is 1s2 2s2 2p2. Therefore, it has four valence electrons which are used for chemical bonding purposes. In most cases, it formed four single covalent bonds. The four single covalent bonds are equivalent and formed by sp3 hybridization.
In the study of chemistry, sp3 hybridization is not the only way to attain noble gas configuration. It can attain octet through multiple covalent bonding with carbon atoms by sp2 or sp hybridization. It also formed multiple bonds with oxygen, nitrogen, or sulfur.
Catenation of Carbon
The property of self-linking of elements through covalent bonds to form long, straight, or branched chains and rings of different sizes is called catenation. The chemistry of carbon is unique due to its catenation property.
It shows maximum catenation in the periodic table elements due to its small size. It enables the nucleus of C-atoms to hold the shared pair of electrons. The strength of C−C single bonds and C−C multiple bonds also helps carbon to form long-chain organic compounds.
Carbon Cycle
It is the biogeochemical cycle where carbon and its compounds are exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of our earth’s environment. Major steps of the carbon cycle may include,
- CO2 and water in our environment are worked up by plants through the agency of chlorophyll and sunlight to form carbohydrates (glucose).
- Some plants are taken by animals and carbon may be bio-accumulated into their bodies.
- When these animals and plants die, carbon may also be released back into our atmosphere.
- Some of the carbon present in dead plants or animals may be stored in the form of coal or petroleum (fossil fuels).
- It releases carbon dioxide into the atmosphere when fossil fuels are burned.
Properties of Carbon
The chemistry of the family of group-14 elements is followed by their electronic configuration but differences in properties between carbon and silicon are rather wide.
| Carbon |
|||
| Symbol | C | ||
| Discovery | Prehistoric | ||
| Name derived from | derived from the Latin word carbo meaning charcoal | ||
| Main allotropes | Diamond, graphite | ||
| Common isotopes | 6C12, 6C13, 6C14 | ||
| Crystal structure | Graphite | Diamond | |
| Simple hexagonal (black) | Face-centered cubic crystal lattice (clear) | ||
| Periodic properties |
|||
| Atomic number | 6 | ||
| Electron per shell | 2, 4 | ||
| Atomic weight | 12.011 | ||
| Electronic configuration | [He] 2s2 2p2 | ||
| Group | 14 | ||
| Period | 2 | ||
| Block | p-block | ||
| Physical properties |
|||
| State at 20 °C | Solid | ||
| Sublimation point | 3825 °C, 6917 °F, 4098 K | ||
| Triple point | 4600 K, 10,800 kPa | ||
| Density (g cm−3) | Diamond | Graphite | |
| 3.513 | 2.267 | ||
| Chemical properties |
|||
| Atomic radius (non-bonded) | 1.70 Å | ||
| Covalent radius | 0.75 Å | ||
| Oxidation number or states | 4, 3, 2, 1, 0, -1, – 2, -3, -4 | ||
| Ionization energy (kJ mol−1) | 1st | 2nd | 3rd |
| 1086.45 | 2352.63 | 4620.47 | |
| Electron affinity | 121.766 kJ mol−1 | ||
| Electronegativity | 2.55 (Pauling scale) | ||
| Molar heat capacity (J mol−1 K−1) |
Diamond | Graphite | |
| 6.155 | 8.517 | ||
| CAS number | 7440-44-0 | ||
Interesting Facts About Carbon
- It can create multiple bonds with other carbon atoms and oxygen, nitrogen, or sulfur atoms.
- Carbon forms negative anions in beryllium carbide (BeC2), sodium alkyl (NaCH3), and alkali metal carbides (Na2C2).
- The electronegativity difference between carbon and fluorine is not sufficient to induce ionic character in fluorocarbon.
- It has the properties of catenation. Therefore, it forms long, straight, or branched chains and rings of different sizes
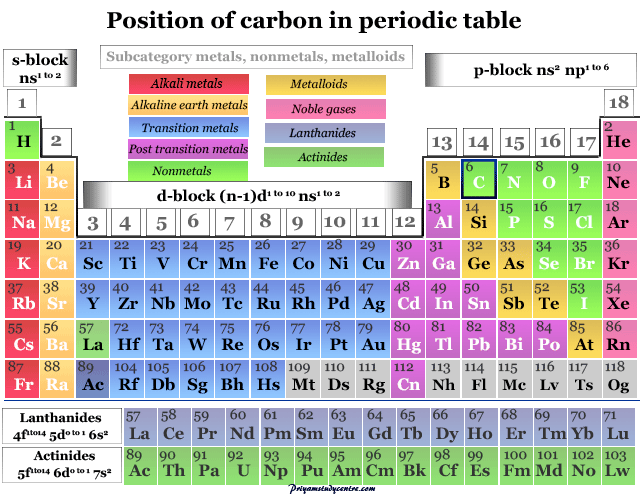
Carbon in Periodic Table
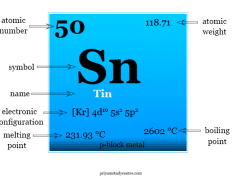
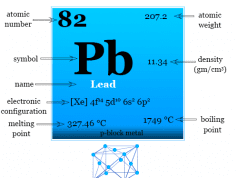
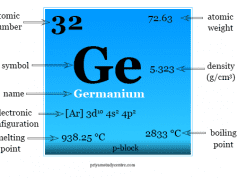
Carbon is placed in group 14 of the periodic table along with the family of silicon, germanium, tin, and lead. All these elements have an outermost quantum shell composition of s2p2.
Sources of Carbon
Carbon is the third most important element after oxygen and hydrogen. Hydrocarbon is the most common combined form of carbon. It is present in the earth’s crust to the extent of about 0.025 percent.
- Carbon is the main component of fuels (wood, kerosine, coal, LPG, CNG, petrol, etc), clothing materials (cotton, nylon, polyester), paper, rubber, plastics, leather, drugs, and dyes.
- Carbon is essential for all living structures. Therefore, it is the main component of all biomolecules such as nucleic acids (DNA and RNA), amino acids, proteins, carbohydrates, and fatty acids.
- Flakes of graphite and crystalline diamond occur in metamorphosed sedimentary rocks like quartz.
Diamond occurs in Kimberlite (a dark color basic rock of Kimberlin found in South Africa) with ancient volcanic pipes. India and Borneo are the oldest diamond-producing countries in the world. The allotrope diamond was found in Brazil in 1729 and South African deposits were discovered in the latter part of the nineteenth century.
Isotopes of Carbon
The principal isotope of carbon is C-12. The isotope, C-12 occurs in nature to the extent of 1.11 percent. It can be used in Fourier transform nuclear magnetic resonance (NMR-spectrum) data analysis.
The atmospheric CO2 contains about 1.2 × 10−10 percent of radioactive C-14 isotope with a half-life of 5570 years. Therefore, The ratio of C-14 and C-12 is used widely in radiocarbon dating or determination of the age of plants or animals. C-14 is also produced by the neutron-proton reaction on nitrogen by thermal neutrons resulting from cosmic radiation.
6C14 → 7N14 + e− + νe + 156.5 keV
Forms of Carbon
Allotopy is the property by which an element exists in more than one form and each has different physical properties but identical chemical properties. These different forms are called allotropes.
Atomic forms of carbon are very short-lived species and are stabilized by various multi-atomic structures known as allotropes. Therefore, it has several crystalline allotropic forms. Diamond and graphite are common allotropic forms of carbon. Other poorly understood allotropes are,
- β-graphite
- Amorphous carbon
- Lonsdaleite or hexagonal diamond
- Chaoite (very rare mineral)
- Fullerenes (Buckyballs)
Carbon Compounds
It is the key element in all living systems. Carbon forms more organic and inorganic compounds than any other chemical element except hydrogen.
The estimated number of carbon compounds known today is about three million or more than three million. Therefore, the question is why carbon can form such a large number of organic and inorganic compounds. The main factors that are responsible for the formation of large numbers of compounds are:
- Catenation: The property of self-linking of C-atoms through covalent bonding to form long, straight, or branched chains and rings of different sizes is called catenation. Carbon can show maximum catenation among periodic table elements due to its small size.
- Tetravelecy of carbon: Carbon belongs to group 14 elements of the periodic table with atomic number 6 and electronic configuration 1s2 2s2 2p2. Therefore, its valency is four and capable of bonding or pairing with four other C-atoms or with atoms of some monovalent chemical elements such as hydrogen and halogens (chlorine, bromine, iodine, etc).
- Tendency to form multiple bonds: Due to the small size of carbon atoms, it tends to form multiple bonds (double and triple bonds) by sharing more than one electron pair. Such type of chemical bond can be formed with its own atoms or with the atoms of elements like oxygen, nitrogen, sulfur, etc.
Bonding of Carbon
The chemistry of carbon compounds can be explained by their electronic configuration. It has atomic number 6 with electronic configuration 1s2 2s2 2p2 and four electrons are present in the outer quantum orbital. Therefore, it can be completed its octet by the following two ways:
- It may gain four electrons from electropositive elements to form C4− anion. However, it would be difficult to hold 10 electrons in an atoms that contain 6 protons in its nucleus.
- It may lose four electrons to form a C4+ cation. However, a large amount of energy is required to remove 4 electrons from C-atom. It is also impossible for C-atom and no compounds known with + 4 cations.
Therefore, to overcome the above problem, carbon can share its valence electrons with other C-atom or atoms of other elements.
In most cases, the compound is formed by four covalent bonding with sp3 hybridization. It not only forms the single covalent bonding to attain the noble gas electronic configuration. It also formed multiple bonds with sp2 and sp-hybridization in unsaturated hydrocarbon compounds like ethylene and acetylene.
Oxidation States of Carbon
The valence shell electronic configuration of C-atom = 2s2 2p2. Therefore, it commonly shows a + 4 oxidation number or state due to the very low electronegativity. However, a −1 oxidation state can also be found in metal carbides such as sodium carbide (Na2C2) or calcium carbide (CaC2).
The common oxidation state of carbon in various inorganic compounds is +4 but the +2 state also forms in CO and transition metal carbonyl complexes. The zero oxidation number of carbon is found in many organic compounds such as formaldehyde (HCHO), dichloromethane (CH2Cl2), glucose (C6H12O6), etc.
Organic Compounds of Carbon
The compounds of carbon except for carbides, oxides, carbonates, and hydrogen carbonate salts are called organic compounds. These compounds were initially extracted from natural substances. Therefore, it can be thought that organic compounds could only form within living organisms (plants and animals).
In 1828, German chemist Fredrich Wohler accidentally prepared urea from ammonium cyanate when he was trying to prepare ammonium cyanate by heating ammonium sulfate and potassium cyanate. Therefore, organic compounds are not only obtained from living organisms (plants and animals) but they also prepared in the laboratory.
All the carbon compounds burn in oxygen to form carbon dioxide and water vapour. Heat and light are also obtained during burning.
C + O2 → CO2 + heat + energy
CH4 + 2 O2 → CO2 + 2 H2O + heat + energy
2 CH3CH2OH + 6 O2 → 4 CO2 + 6 H2O + heat + energy
Various allotropic forms of carbon and its compounds ignite, they keep on burning without the requirement of any other additional energy. Therefore, these compounds are called fuels and are used widely in cooking food, and running machines in factories.
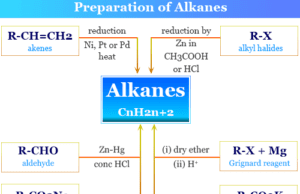
Production Process
Production of Coke
Among all the natural forms, coke is used in large quantities for energy generation in our daily lives. Such forms are grey, hard, and porous coal-based fuels that have high carbon content and few impurities.
Coke is obtained by cooking or high-temperature carbonization of coal in the absence of air. Generally, it can be made by heating coal or oil in the absence of air or by a destructive distillation process. Coke is an important industrial product that is used mainly smelting of iron ore and steel.
F3O4 + 3 C (coke) + 2 O2 → 3Fe + 4 CO2
We used both coke and coal as fuels but coke is considered to be a better fuel than coal because coke has been higher calorific value and does not produce smoke on burning or lower impact on air pollution.
Production of Graphite
Natural graphite was obtained in a mixture of mica, quartz, and silicates. The mixture was washed by flotation and heated with hydrochloric acid and hydrofluoric acid in a vacuum. The residual silicon compounds precipitated as SiF4 molecules.
Nearly half of the industrial requirement of graphite is made by the synthetic process. Therefore, it is made by heating silica with coke in an electric furnace at 2500 °C for about 24 hours.
SiO2 + 3 C → SiC + 2 CO + C (graphite) + Si
Production of Diamond
Natural diamond is mined in large quantities which are about 18 tonnes per year. Of these 30% are used as gems while the rest goes to various industries.
Only small-size industrial diamonds are made artificially by subjecting graphite to 125000 atm pressure and 3000 K temperature. But if we use the metal chemical catalyst, the conversion is achieved at 70,000 atm pressure and 2000 K temperature.
Carbon Black
Carbon black is prepared by incomplete thermodynamics combustion of hydrocarbons in a limited supply of air. It can be produced mainly by the incomplete combustion of coal tar, vegetable material, or petroleum products, including fuel oil, fluid catalytic cracking tar, etc. It is a para-crystalline form of carbon that has a high surface-area-to-volume ratio.
Carbon black can be used mainly for making automobile tires and black pigment. It is a common conductive additive for lithium-ion electrochemical cells.
Activated Charcoal
Activated charcoal or activated carbon is prepared by controlling the pyrolysis of organic compounds like sawdust or coconut shells.
Activation of the surface is accompanied by adding materials that oxidize or dehydrate the organic substrate on the surface. The chemicals commonly used for the activation of charcoal are phosphoric acid, potassium hydroxide, sodium hydroxide, potassium carbonate, calcium chloride, zinc chloride, etc.
The edge of the hexagonal graphite sheets is probably covered with oxygenated groups which are responsible for surface activity.
Graphite Fibers
Graphite fibers are obtained when synthetic fibers or asphaltic fibers are subjected to pyrolysis. The strong fibers have the same structural formula as graphite but they contain layers of ribbons in place of sheets. It is parallel to the axis of fibers.
The strong bonds in a plane are responsible for high tensile strength. Graphite fibers are used mainly for making tennis rackets, aircraft components, etc.
Uses of Carbon
The first two elements (carbon and silicon) in the group 14 family occupy a special position in our lives. Therefore, they are used widely in our daily lives in various production processes.
- Hydrocarbon is the major economic form of carbon that occurs in nature as fossil fuels like methane gas and crude oil or petroleum. Therefore, crude oil is used for the production of gasoline, petrol, diesel, kerosene, and many other petrochemical products.
- Cellulose is a carbon-containing polymer obtained from plants. It is used for making wool, cotton, and linen. It also maintains the structure of plants.
- Plastics are synthetic polymers of carbon. It is used widely in our daily life.
- The allotrope coke is vital in the extraction of iron and many other metals.
Uses of Graphite and Diamond
Graphites and diamonds are two major allotropic forms of carbon. Diamond is a colourless transparent substance with extraordinary brilliance due to its high refractive index while graphite is a good conductor of electricity due to the presence of free electrons.
Uses of Graphite
- Graphite is used mainly in steelmaking, metal foundries, refractories, making crucibles, nozzles, fuel cell electrodes, etc.
- It is used for making lubricants.
- It is also used in brake lining, pencils, brushes for electric motors, etc.
- Graphite is used as a neutron moderator in nuclear power reactors.
Uses of Diamond
The useable diamonds are classified into two classes, gem-grade diamonds, and industrial-grade diamonds. About 80% of mined diamonds are unsuitable for making gemstones. Therefore, they are relegated to industrial use.
- Like gold or platinum, gem-grade diamonds is a valuable gemstone.
- Industrially, diamond is used mainly for cutting, drilling, grinding, and polishing.
Uses of Carbon Black
- Carbon black is largely used in the rubber industry to increase the strength of rubber. These rubbers are used particularly for making car tires and plastic compounds.
- It is a common conductive additive for lithium-ion chemical cells.
- Vegetable bases carbon black can be used for the colouring of food materials.
- It is a black pigment that is used widely in printing ink, carbon paper, automotive finishes, and laser printer toner.
Uses of Activated Charcoal
- Activated charcoal is a very efficient absorbent used for absorbing organic pollutants from drinking water and greenhouse gases from the air.
- It is largely used as a decolorizing agent in the sugar industry.
- In medicinal chemistry, activated charcoal is used to absorb toxins, poisons, or gases from the digestive system.
- It is used in gas purification systems, including air pollution and gas masks.
- Activated carbon is also used as a chemical catalyst in the sewage water pollution system.
- In analytical chemistry, activated carbon can also be used as a stationary phase in low-pressure chromatographic separation of carbohydrates by using ethanol solutions as a mobile phase.