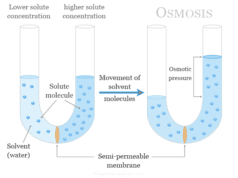
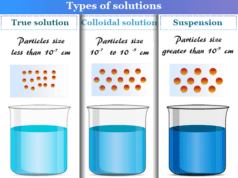
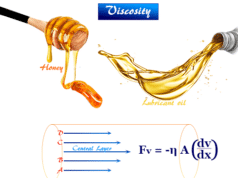
Emulsion Definition in Chemistry
Emulsion in chemistry is formed when a liquid is dispersed in another liquid that is not miscible to each other. Milk is a bright example of an emulsion solution where fats are dispersed in the medium of water by emulsifying agents like protein casein. It is a two phase system of matter like colloids. Oil in water and water in oil are two types of emulsions where oil is dispersed in the medium of water and water is dispersed in the medium of oil. The two types of emulsions are generally identified by pouring water on them. In oil in water type emulsion, water is soluble in the solution while in the water in oil type emulsion, oil is soluble in the solution. Sometimes, an electrolyte is added to the emulsion, and the conductance is found to increase, then it is identified as oil in water type.

Types of Emulsion
Emulsion is classified into two types,
- Oil in water type: In oil in water type emulsion, oil is dispersed in medium of water.
- Water in oil type: In the water in oil type emulsion, water is dispersed in the medium of oil.
Here, oil is synonymous with any liquid which is immiscible with water.
Emulsifying Agent
Generally, an emulsion is stabilized by introducing third substances like emulsifying agents. These agents are three types,
- Soap and detergents
- Lyophilic sols
- Insoluble powder
Soap and Detergents
These are the sodium or potassium salts of long-chain fatty acids and sulphonates. They are surfactants that reduce the surface tension between a liquid and another medium and are used as emulsification agents.
Lyophilic Sols
Lyophilic sols are very stable emulsifying agents that have strong interaction forces between the dispersed phase and dispersion medium. Rubber, starch, proteins, albumin, and gelatine are examples of lyophilic emulsifying agents.
Insoluble Powder
These are basic sulfates of iron, copper, or nickel, lead sulfate (PbSO4), iron (III) oxide or ferric oxide (Fe2O3), etc. The emulsifying agents tend to decrease the surface tension at the oil-water interface due to the formation of globules or droplets.
Milk is an emulsion of fat in water (oil in water type) and stabilizes by emulsifying agent casein protein.
Properties of Emulsion
The behavior of emulsion in the absence of an emulsifying agent resembles that of lyophobic sols. They generally show the Tyndall effect, Brownian motion, low viscosity, electrophoresis, etc. The globules move towards the electrode (anode) at the mobility of 1 × 10−4 cm2 sec−1 volt−1.
The behavior of emulsion in the presence of an emulsifying agent resembles that of lyophilic sols. They are generally highly viscous and insensitive to electrolytes. Chemical destruction of emulsifying agents destabilizes the emulsions and it is called de-emulsification.
If soap or detergents are used as emulsifying agents, they can be destroyed by hydrolysis. The physical method like centrifuge or getting used to destroy it.
Mechanism of Emulsification
An emulsifying agent decreases the surface tension at the oil-water interface. However, it is found that the nature of the substances plays a great role in the formation of different types of emulsions.
For example, if sodium-oleate is used, it becomes oil in water type. When calcium-oleate is used, the emulsification becomes water in the oil type.
The fact can be explained in the following way. Sodium-oleate is preferably dissolved in water and the surface tension of water decreases facilitating the formation of oil in the water-type emulsion.