Activation Energy Definition
Activation energy in chemistry or biology defines a certain amount of energy acquired by the atoms or molecule to be active before the chemical transformation or activated complex formation. Before the chemical reaction proceeds, the reactant molecules must be in an energy-rich species or activated state. Activation energy theory or formula derived from the Arrhenius equation suggests that the efficiency of enzymes is greater than chemical catalysts. According to Arrhenius when the reactants are converted into products, the reactant has passed through the crucial configuration or activation to higher levels. This is called the transition state.
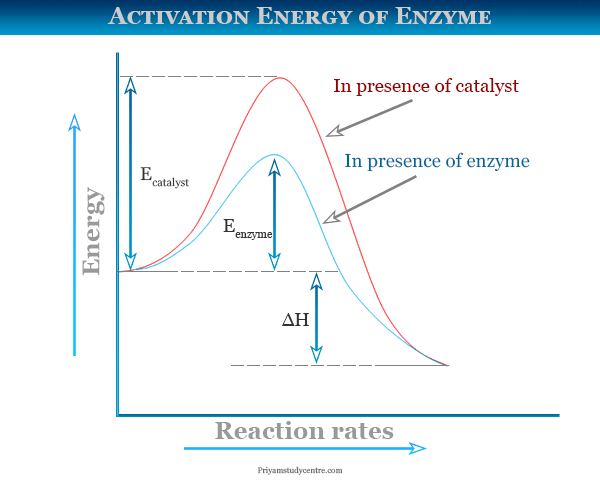
Activation Energy and Enzymes
Like other chemical catalysts, enzymes lower the activation energy of the reaction that they catalyze. They are far more efficient than chemical catalysts because enzymes lower the activation energy to a much greater extent.
For example, the Ea value for the decomposition of hydrogen peroxide by platinum = 50.2 kJ mol−1. When the same reaction proceeds by enzyme catalase, Ea = 2.5 kJ mol−1. The factor which accounts for the high efficiency of enzyme-catalyzed reactions are,
- The binding of the reactant molecules to enzymes increases the concentration of reactant molecules. Therefore, the transition state reaches more readily.
- Binding produces a strain effect in reactant molecules which lowers the activation energy of the reaction.
Activated Complex Theory
The activated complex theory is also known as the theory of absolute reaction rate proposed by Arrhenius.
According to Arrhenius when the reactants are converted into products, the reactant has passed through the crucial configuration or activation to higher levels. This is called the transition state.
In the transition state, the reactants form an activated complex whose concentration can be calculated from the concentration of the reactant.
Examples of Activated Complex
The idea of the activated complex in the transition state is better understood by the example of the formation of a molecule like hydrogen iodide.
Suppose a hydrogen atom approaches halogen molecules to form an activated complex. When these two are far apart, the energy of the system is equal to the sum of individual energies.
As the hydrogen atom comes close to the iodine molecule, the orbitals of the hydrogen and iodine become overlap. Therefore, the iodine-iodine chemical bonding begins to stretch. The energy of the system now trends to activated or increases by the formation of the complex.
Activation Energy Diagram
In learning chemistry, the meaning of activation energy or activated complex is made clear with the schematic diagram or reaction rate coordinate vs energy graph given in the below picture,
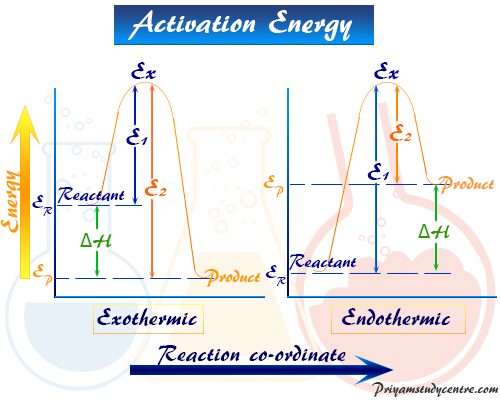
The average energy of the reactant and product in the above diagram is represented by ER and EP respectively. There is minimum energy for the reaction denoted by Ex required for any chemical changes.
From the above diagram, the excess or additional energy (Ex − ER) that the reactant must acquire for a chemical transformation. It is called activation energy in chemical kinetics. It is denoted by E1 for atoms or molecules.
After the chemical transformation, the product has an average free energy EB. Therefore, the amount of energy such as (Ex − EB) = E2 is calculated from the Van’t Hoff equation for any chemical transformation.
- If E2 > E1, the reaction is exothermic.
- When E2 < E1, the chemical transformation is endothermic.
Arrhenius Formula for Activation Energy
Activation energy is usually represented by Ea and is found in the Arrhenius mathematical formula,
k = A e−Ea/RT
where A = constant
It is obvious that the Ea in the Arrhenius equation must have the units of energy. Generally, it is measured by units like joules per mole (J/mol), kilojoules per mole (kJ/mol), or kilocalories per mole (kcal/mol).
In enzyme catalysis reactions in biology, the chemical catalyst can lower the activation energy which permits a larger amount of reaction in a given time by the formation of the intermediate activated complex.