Nickel Metal
Nickel is a chemical element, silvery, malleable, ductile, ferromagnetic transition metal of group 10 of the periodic table with atomic number 28 and symbol Ni. It is used in making alloys with iron and other nonferrous metals like silver and chromium. The valence shell electronic configuration of nickel [Ar] 3d8 4s2 with incomplete d-orbital. Therefore, it commonly shows a +2 oxidation number or state. The compact metal is quite resistant to air or water but finely divided nickel is very reactive and pyrophoric. It is slowly attacked by dilute hydrochloric acid and sulfuric acid but dissolves readily in dilute nitric acid and aqua regia. The face-centered cubic crystal lattice nickel is a hard silvery metal. It is very malleable and ductile and easily rolled down or polished.
History of Nickel
The name of the metal nickel is derived from the German word devil spirit. In the 17th century, a mineral had the appearance of a cuprite but could not be melted to obtain copper. Metallurgists decided that the ore was dominated by the evil spirit of the mountain copper devil or Kupfer-nickel.
The confusion regarding the mineral persists until 1751. In 1751, Axel Fredrik Cronstedt extracted copper from the copper devil or Kupfer-nickel mineral in Sweden.
Sometime after, scientists believed that Ni was a mixture of cobalt, iron, arsenic, and copper. The doubts were finally resolved by scientist T Bergmann in 1775.
Properties of Nickel
The ferromagnetic properties and Courie point of cobalt (375 °C) are less than that of cobalt and iron. The compact metal is quite resistant to air and water but strong heating in the air produces little amount of oxide.
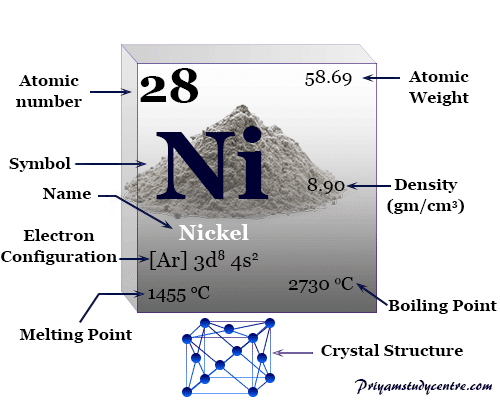
Some common physical and atomic properties of nickel are given below in the table,
| Nickel | |||
| Symbol | Ni | ||
| Discovery | Axel Fredrik Cronstedt in 1751 | ||
| Name derived from | The German word devil spirit | ||
| Common isotope | 28Ni58 | ||
| Oxidation states | +3, +2, 0 | ||
| CAS number | 7440-02-0 | ||
| Periodic properties | |||
| Atomic number | 28 | ||
| Relative atomic mass | 58.693 | ||
| Electron per cell | 2, 8, 16, 2 | ||
| Electronic Configuration | [Ar] 3d8 4s2 | ||
| Block | d-block | ||
| Group | 10 | ||
| Period | 4 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 1455 °C, 2651 °F, 1728 K | ||
| Boiling point | 2913 °C, 5275 °F, 3186 K | ||
| Molar heat capacity | 26.07 J mol−1 K−1 | ||
| Crystal structure | face-centered cubic (fcc) | ||
| Density | 8.90 g/cm3 | ||
| Electrical resistivity | 69.3 nΩ m | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 1.97 Å | ||
| Covalent radius | 1.17 Å | ||
| Electronegativity | 1.91 (Pauling scale) | ||
| Electron affinity | 111.54 kJ mol−1 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 737.13 | 1753.03 | 3395.32 | |
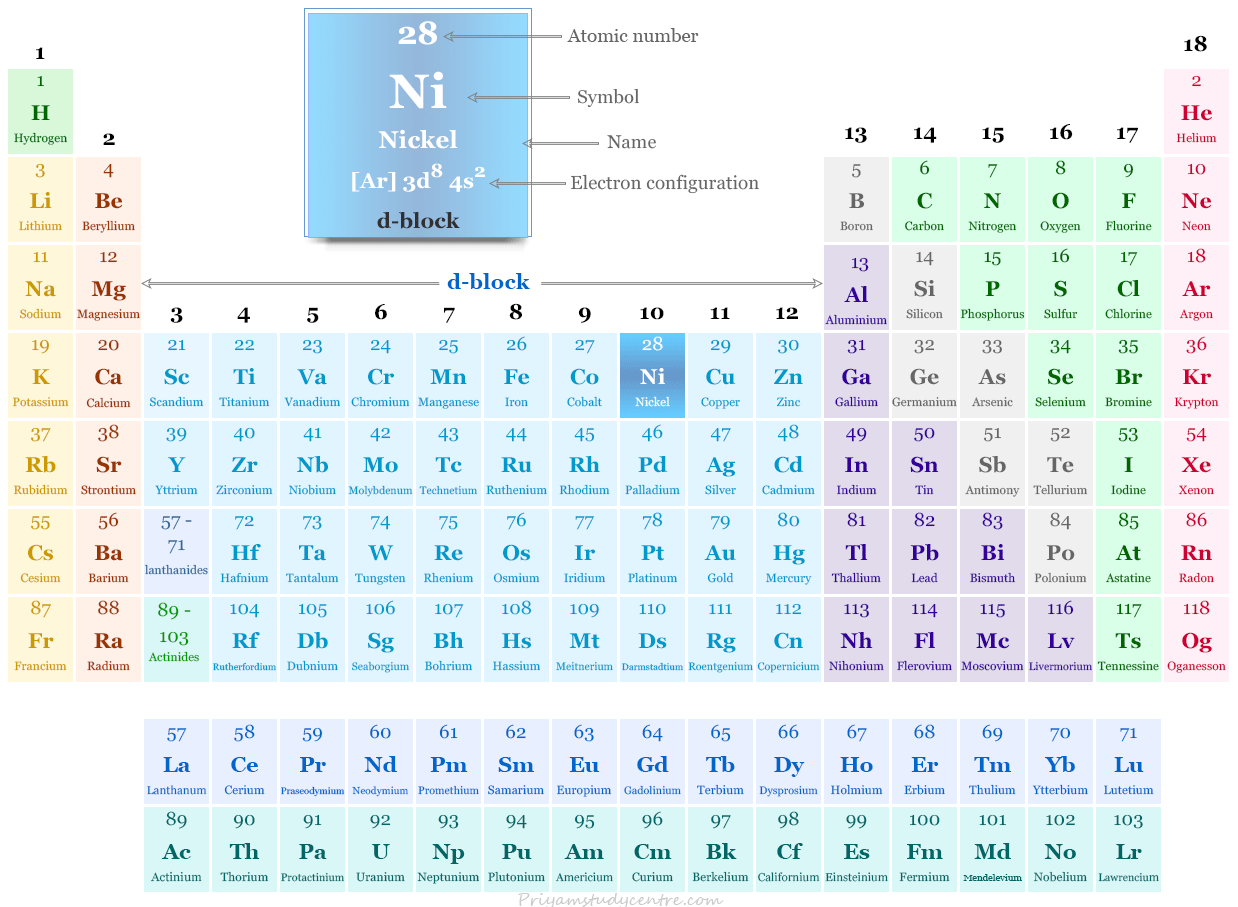
Nickel in the Periodic Table
The transition metal nickel is placed in period-4 and group-10 of the periodic table along with other group members palladium and platinum.
Where is Nickel Found?
It is the twenty-second most abundant element found in the earth’s crust and the seventh most abundant metal among the transition elements. It is found mostly in combination with sulfur, iron, and arsenic in the mineral-like nickeline.
The nickeliferous limonite, NiO(OH) or FeO(OH), and garnierite (Ni, Mg)6Si4O10(OH)8 are the main ores of the metal.
Indonesia and Australia have the biggest reserves of metal. Garnierite is found in New Caledonia, Cuba, Brazil, and Queensland. However, the most important single deposit of nickel is found in countries like Sudbury, Canada, Russia, and South Africa.
Isotopes of Nickel
It has five naturally occurring isotopes 58Ni, 60Ni, 61Ni, 62Ni, and 64Ni with several radioactive isotopes (atomic mass ranges 48 to 78).
Production of Nickel
Nickel is generally produced from sulfide ore which contains about 3 percent of Ni, 1.5 percent of copper, and precious metals like gold, silver, and platinum. The main steps for the extraction of metal,
- Concentrated sulfide ore is magnetically or selective floatation.
- The concentrated sulfide ore is subject to a series of roasting and smelting with the addition of silica and limestone.
- The mate-rich Ni and Cu sulfide are generally separated by solid phase which is then extracted by the Mond process or electrolytically.
Mond Process for Refining
- In the mond process for refining nickel, the Ni-Cu sulfide is roasted to oxidize to their oxides.
- The oxides leach with hot dilute sulfuric acid, the most of the copper oxide dissolves out as CuSO4.
- The oxide is now reduced with hydrogen in water gas at about 300 to 350 °C.
- The impure metal is now converted to volatile tetracrbonyl by the action of carbon monoxide at atmospheric pressure of around 50 °C.
- The pure form of nickel is obtained by decomposing metal tetracarbonyl.
Electrolytic Refining
During the electrolytic refining process, the Ni-Cu mate is roasted in a multiple hearth roaster to form their respective oxides. Most of the copper oxide is leached out with hot dilute sulfuric acid.
The residue is further reduced with coke which contains 65 percent of Ni, 30 percent of copper, and 3 to 8 percent of sulfur and iron residue.
Electrolysis is carried out with NiSO4 solution with Ni electrodes or cathodes on which the pure metal is deposited.
Interesting Facts About Nickel
- It is slowly attacked by dilute hydrochloric acid and sulfuric acid but is readily dissolved in nitric acid.
- Practically, it is unattacked by caustic alkalies.
- It reacts slowly with fluorine at ordinary temperatures. However, heated Ni catches fire in chlorine or bromine.
- It has a ferromagnetic property that is less than that of iron or cobalt.
Chemical Compounds
In the +2 state, nickel forms a wide range of simple compounds like oxide (NiO), hydroxide (NiOH), halides sulfide (NiS), carbonate (NiCO3), cyanide (NiCN), and complex compounds.
Some common compounds of nickel are discussed below,
Nickel Oxide
Green solid NiO is the common oxide of nickel. NiO is formed during the thermal decomposition of the hydroxide, carbonate, oxalate, or nitrate.
It has a cubic crystalline solid structure that is readily soluble in water.
Hydroxide
The green color nickel hydroxide has the chemical formula Ni(OH)2. The green gel precipitate of Ni(OH)2 is formed during the addition of an alkali metal hydroxide solution to aqueous Ni (II).
The precipitate turns crystalline on prolonged standing. Ni(OH)2 dissolved readily in acids and ammonia to form soluble complex compounds.
Halides
It is the only metal from the first transition elements that form halides in a +2 oxidation state. Therefore, all four anhydrous halides like NiX2 are known.
Nickel fluoride (NiF2) is moderately soluble in water but other halides are fairly soluble in water.
Uses of Nickel
The metal is used for making electrical cells like alkaline Fe-Ni and Cd-Ni cells and gas diffusion electrodes for alkaline fuel cells.
What is Nickel Alloy?
The metal is used mostly in the form of alloys like Monel metal, nickel silver, nichrome, alnico with iron, and other nonferrous metals.
A small part of Ni increases the quality of cast iron. For example, 0.25 to 0.45 percent of Ni in steel increases the mechanical properties and corrosion resistance properties.
68 percent of nickel from global production uses for making stainless steel. The metal is also used in many industrial and consumer products like stainless steel, magnets, rechargeable batteries, electrical plating, etc.
The alloy, alnico is used for making permanent magnets like horseshoe magnets.
Nickel Catalyst Uses
The chemical catalyst of nickel is used for the hydrogenation of oils and hydrocarbon in organic chemistry.
The common chemical catalyst for this purpose is Raney Nickel. It is a highly active form of the metal prepared by dissolving aluminum from the alloy NiAl3 with alkali.
Role of Nickel in Biological System
The natural biological application of nickel was established only in 1975 with the discovery of the metal in the enzyme jack bean urease.
The enzyme is present in a number of plants, eubacteria, archaebacteria, and fungi. The enzyme contains an uncommon oxidation state like +2 with a redox reaction inactive unit containing octahedral Ni which acts as a Lewis acid.
The redox-active nickel centers are present in other enzymes like Hydrogenase, CO dehydrogenase, and methyl-S-coenzyme M reductase found mostly in bacteria.