Crystalline Solids and Amorphous Solids
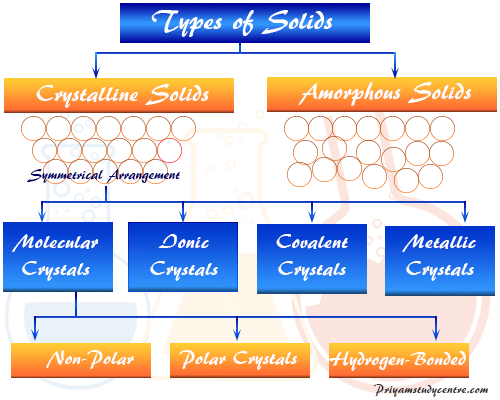
Crystalline solids and amorphous solids are the two types of solid materials formed by atoms, ions, or molecules. Molecular, ionic, covalent, and metallic crystalline solids are the main types of crystals formed by their constituents with a definite structure. A crystal or crystalline solid is a material where constituents are arranged in a definite and orderly arrangement over a large distance but an amorphous solid is a material that does not possess a definite structure or sharp melting point, and the constituents do not form an order arrangement. Sodium chloride and potassium chloride are the most common examples of crystalline solids but glass, pitch, rubber, and plastics are common examples of amorphous solid materials that we use widely in our daily life. In chemistry, a solid molecule is characterized by its definite shape, strength, density, and rigidity rather than liquid and gas molecules.
Solids are rigid due to the absence of translatory motion on the structural unit. These units are fixed to their mean position with strong forces of attraction.
Crystalline Solid
A crystal or crystalline solid is a solid material where constituents like atoms, molecules, or ions are arranged in a definite and orderly arrangement over a large distance in the crystal lattice. This is called a long-range order.
Examples of Crystalline Solid
Lithium chloride, sodium chloride, potassium chloride, sugar, ice, and quartz are the most common examples of crystalline solid. These materials contain a definite geometrical structural arrangement and are also melted by a specific heat.
Structure of Crystalline Solids
Crystalline solids have well-defined edges and faces with definite melting points. The study of the geometrical structure in the crystal lattice is called crystallography.
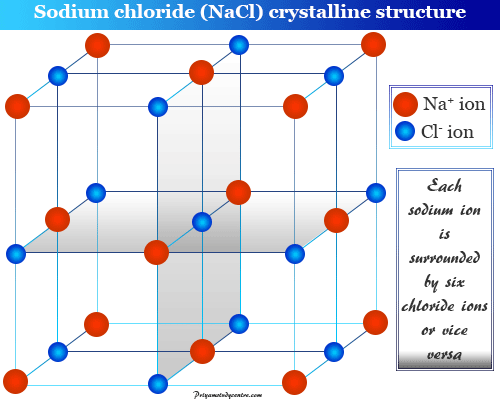
The structure of the sodium chloride crystal is given below the picture,
Bragg diffraction experiments in physics or chemistry are very useful for the determination and analysis of crystal structure. It is developed from the very simple relation between wavelengths of the X-ray radiation and spacing between the two lattice planes.
Properties of Crystalline Solids
- The constituents in crystalline solids may be atoms, ions, or molecules.
- Crystalline solids also have the properties of sharp melting points, flat faces, and sharp edges.
- It is a well-developed form that is arranged symmetrically.
- Definite and the ordered arrangement of the constituents extends over a large distance in crystal lattices.
- Crystalline solids belonging to the cubic class mostly show anisotropic properties. However, the magnitude of the anisotropic characteristics depends on the direction of structure measurement.
Amorphous Solid
An amorphous solid is a material that does not possess a definite structure or sharp melting point, and the constituents do not form an order arrangement. Therefore, the constituents extend over a short distance. This is called short-range order.
Examples of Amorphous Solids
Glass, pitch, rubber, and plastics are common examples of amorphous solids. It has many characteristics of crystalline solid such as shape, rigidity, and hardness.
They are not arranged orderly and melt gradually over a range of temperatures. Therefore, amorphous solids like Glass, pitch, rubber, and plastics are called supercooled liquids rather than solids.
Difference Between Crystalline and Amorphous Solids
The main differences between crystalline and amorphous solids are,
- Crystalline solids have a definite structure with a sharp melting point. On the other hand, amorphous solids do not contain a definite structure with a sharp melting point.
- The constituents of crystalline solids are orderly arranged over a long range in the crystal lattice. However, in an amorphous solid, the constituents do not have an order arrangement.
Types of Crystalline Solids
Based on the nature of force operating between constituents (atoms, ions, molecules), the crystalline solids or crystals are classified into four types,
- Molecular crystalline solid
- Ionic crystalline solid
- Covalent crystals
- Metallic crystalline solid
Molecular Crystalline Solids
Forces that hold the constituents of molecular crystalline solid are weak Van der Waals type. The forces are two types−interatomic or intermolecular and originate from the dipole-dipole attraction.
Due to weaker forces, the molecular crystals have a soft and comparatively low melting point. The common examples of molecular crystalline solids are carbon dioxide, nitrogen, mica, borax, boric acid, and most organic hydrocarbons like alkanes or alkenes.
According to polarity, the molecular crystalline solids are mainly three types,
- Non-polar molecules
- Polar molecules
- Hydrogen bonding molecules
Non-Polar Molecules
Some molecular crystalline solid have a non-directional structure due to the absence of the dipole moment.
Hydrogen, helium, argon, oxygen, chlorine, carbon dioxide, and methane molecules are examples of non-polar crystalline solid molecules. The forces operating between constituents are a weak London dispersion force.
Polar Molecules
A polar molecule is one in which one end of the molecule contains a positive charge and the other end contains a negative charge. Sulfur dioxide and ammonia are common examples of polar crystalline molecules. Due to electric polarization, the constituent elements are bound by low forces of attraction.
Hydrogen Bonding Molecules
Hydrogen bonding is a weak type of chemical bonding due to very unstable attractive forces between a hydrogen atom and electronegative atoms like oxygen, nitrogen, and fluorine. Such a type of bonding can be seen commonly in the dimer of formic acid or acetic acid.
The hydrogen bond is electrostatic but very weak with a bond energy of 5 to 6 kcal. The most discussed example of a hydrogen-bonding crystalline solid is ice.
In ice, the oxygen atom is surrounded by four hydrogen atoms at the corner of the tetrahedron. A list of organic materials like alcohol, carboxylic acid, and proteins are the class of hydrogen-bonding crystalline solids.
Ionic Crystalline Solids
Forces involved in an ionic crystalline solid are electrostatic in nature. These are strong and non-directional types. Therefore, an ionic crystalline solid is strong and likely to be brittle.
They have very little elasticity and cannot be bent easily. The melting point in ionic crystals is also high, which decreases with increasing the size of the ions.
Examples of Ionic Crystalline Solids
Sodium chloride (NaCl), potassium chloride (KCl), and magnesium chloride (MgCl2), calcium oxide are the most common examples of ionic crystals.
Calcium carbonate (CaCO3) is an example of an ionic crystal where covalent bonding holds some atoms together.
Covalent Crystals
In many crystals, the atoms in the structural units are held together by covalent bonding by pairing electrons of hybridized orbitals to form giant-type molecules.
Examples of Covalent Crystals
Diamonds, germanium, zinc sulfide, silver iodide, and silicon carbide are known examples of covalent crystals. In a diamond, every carbon atom covalently links with the other four carbon atoms along the tetrahedron.
Metallic Crystalline Solids
Electrons are generally held loosely bound in these types of crystalline solids. They are mostly good conductors of electrical energy and thermal energy. Metallic crystalline solid is strong but can be bent.
The metal atom mostly formed cubic body-centered, face-centered, and hexagonal closed-packed crystalline solids.
In hexagonal crystalline form, every metal atom is surrounded by 12 other metal atoms by the metallic bond with coordination number twelve. The forces are non-directional in nature. All the metals of periodic table elements are generally formed by different types of crystal structures.
Crystalline Allotropes of Carbon
Allotropes of carbon have formed both types of solids, crystalline and amorphous in nature.
Diamond, Lonsdaleite, and graphite are examples of crystalline allotropes of carbon. However, the other rare and poorly understood allotropes of carbon are β-graphite, hexagonal diamond, Chaoite (a very rare mineral), and carbon VI.
Structure of Diamond
Diamond is a colourless transparent substance with extraordinary brilliance due to its high refractive index. Due absence of free electrons, it does not conduct electricity but it has high thermal conductivity and a high melting point. A lot of energy is required to break the network of strong covalent bonds in the diamond crystal.
In diamond, each sp3 hybridized orbital of carbon is tetrahedrally surrounded by four other carbon atoms. It is a giant type molecule where each carbon atom bonded to four other carbon atoms to form a rigid three-dimensional structure. Therefore, a diamond is quite heavy and extremely hard.
The C−C bond distance in the diamond crystal is 154 pm. These tetrahedral structures form a cubic crystal unit of the diamond.
Structure of Graphite
Graphite consists of the layer structure in each layer of the carbon atoms is sp2 hybridized. Therefore, each carbon atom in a graphite layer is joined to three other carbon atoms by strong covalent bonds to form a flat hexagonal ring. However, the fourth electron in each carbon atom is free. Therefore, graphite is arranged in a hexagonal planner arrangement with free π-electrons.
The π-electrons are responsible for the electrical conductance in graphite. Successive layers of carbon atoms attached by weak van der Waals forces with separation of layers 335 pm. Therefore, these layers can slide over one another and it makes graphite slippy in touch.
Amorphous Allotropes of Carbon
Coal, carbon black, soot, carbide-derived carbon, and other impure forms rather than graphite and diamond are examples of amorphous solids.
All of these forms contain poly-crystalline structures. Therefore, amorphous carbon is the allotropes of carbon that do not form a crystalline solid structure.
Frequently Asked Questions
What is Crystalline and Amorphous Solids?
Crystalline solids are true solids that contain well-defined edges and faces, diffract X-rays, and have sharp melting points but amorphous solids do not possess a definite structure or order arrangement and sharp melting points (melt over a wide range of temperatures).
Quartz, sugar, diamonds, rock, sodium chloride, potassium chloride, calcium fluoride, silicon dioxide, and alum are the common crystalline forms found in nature. Similarly, glass, pitch, rubber, and plastics are common examples of amorphous solids that are used widely to fulfill our daily needs.
What are the 7 types of crystals?
Seven types of crystal structures are triclinic, monoclinic, orthorhombic, tetragonal, trigonal, hexagonal, and cubic crystals.
What is the allotropy of carbon?
Allotopy is a property where an element exists in more than one form and each form has different physical properties but identical chemical properties. Carbon exists in different allotropic forms in nature.
- Crystalline form: diamond, graphite, and fullerene.
- Micro-crystalline or amorphous form: coal, lampblack, and charcoal.
What is an amorphous solid?
An amorphous solid is any noncrystalline solid material where constituent atoms and molecules are not organized in a definite lattice pattern. Unlike a crystalline solid, amorphous material melts gradually over a range of temperatures.