Copper Element
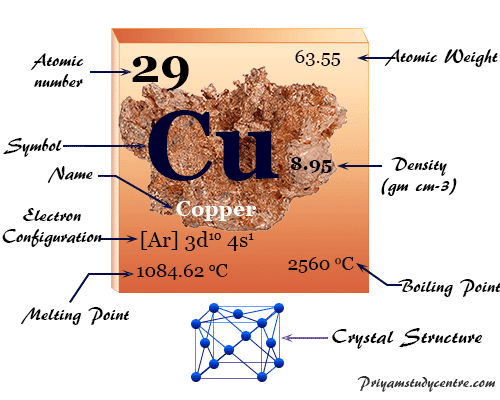
Copper is a chemical element or reddish-brown transition metal of Group 11 or IB of the periodic table with the symbol Cu and atomic number 29. It is a soft, tough, and lustrous metal with high thermal and electrical conductance. In chemistry, the physical and chemical properties of coinage or noble metals like copper, silver, and gold are very similar. Copper element helps in the formation of hemoglobin (an oxygen carrier) in the animal body. Copper metal is also found in hemocyanin in the blood of cuttlefish and plants like green peas. The metal forms a face-centered cubic crystal lattice. It is found in our environment as a free metallic state or ores which can be easily extracted. Bronze, the alloy of copper and tin has been used in history by mankind since the Bronze Age.
Who Discovered Copper?
The archaeological discovery of the island, namely Cyprus in the eastern Mediterranean Sea is famous for its mines of copper. The name copper is derived from the Latin name Cuprum via Cyprium.
Properties of Copper
| Copper |
|||
| Chemical symbol | Cu | ||
| Discovery | Historic age | ||
| Name derived from | The old English word coper and from the Latin word Cyprium aes meaning a metal from Cyprus | ||
| Main isotope | 29Cu63 | ||
| Oxidation states | +1 and +2 | ||
| CAS number | 7440-50-8 | ||
| Periodic properties |
|||
| Atomic number | 29 | ||
| Atomic weight | 63.546 | ||
| Electron per shell | 2, 8, 18, 1 | ||
| Electronic configuration | [Ar] 3d10 4s1 | ||
| Group | 11 | ||
| Period | 4 | ||
| Block | d-block | ||
| Physical properties |
|||
| State at 20 °C | Solid | ||
| Meting point | 1084.6 °C | ||
| Boiling point | 2560 °C | ||
| Density | 8.95 gm/cm3 | ||
| Molar heat capacity | 24.440 J mol−1 K−1 | ||
| Crystal structure | face-centered cubic (fcc) | ||
| Electrical resistivity | 16.78 nΩ m | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 1.96 Å | ||
| Covalent radius | 1.22 Å | ||
| Electronegativity | 1.90 (Pauling scale) | ||
| Electron affinity | 119.16 kJ mol−1 | ||
| Ionization energies (kJ mol−1) | 1st | 2nd | 3rd |
| 745.48 | 1957.92 | 3554.61 | |
Copper in the Periodic Table
The currency metals, Cu, Ag, Au are placed in group-11 or group-1B of the periodic table. All these metals contain ns1 (n−1)d10 valence shell electronic configuration with filled d subshell. Due to the presence of incompletely filled d-subshell in +2 or +3 oxidation number or state, they are called transition metals.
Chemical Properties
In the early Mendeleev periodic table, coinage metals like Cu, Ag, and Au are placed with alkali metals. Both the alkali metal and the currency or coinage metal have one electron in the outermost s-orbital and +1 is the common oxidation number or state. However, the physical and chemical properties of these two families vary widely.
The alkali metals are soft, low melting highly electropositive elements while coinage or noble metals are relatively high melting and comparatively less electropositive metals. Therefore, coinage metals are placed in group 11 on the modern periodic table.
The group 11 metals have high first ionization energy and smaller ionic radii. The involvement of d-electrons in metallic bonding impacts a higher melting point of copper.
The Cu (II) ion is more stable than the Cu (I) ion in a water solution. It is due to the small size and hence the enormously high hydration energy of Cu (II) over that of Cu (I) which offsets the second ionization energy of copper.
Where is Copper Found?
Copper is present in the earth’s crust to the extent of 68 ppm which is slightly less than that of nickel.
The native copper (minerals of basaltic leaves) and the reduced form of metal compounds like sulfide, carbonates, arsenide, and chloride are found in various locations of the world.
It is largely found in North or South America, Chile (the world’s leading producer), Congo, and Russian counties. In India, two main deposits of Cu ore are present in Bihar (Singhbhum) and Rajasthan (Alwar, Ajmer, and Khetri).
Copper Ores
The major ores of the metal are given below the table,
| Copper ore | Chemical formula |
| Chalcopyrite | CuFeS2 |
| Cuprite | Cu2O |
| Covellite | CuS |
| Chalcocite | Cu2S |
| Malachite | [CuCO3, Cu(OH)2] |
| Bornite | 2 Cu2S, CuS, FeS |
| Digenite | Cu9S5 |
| Dioptase | CuSiO2(OH)2 |
Copper Extraction Process
It is extracted mainly from the sulfide ore by the self-reduction process. The low grade ( 0.5 percent Cu) converts to a high grade (15 to 20 percent Cu) for the high production of the metal. The froth flotation process is used for this purpose.
The main steps for extraction of copper are,
Smelting of Copper
The ore is roasting and smelting (about 1400 °C) with the addition of silica in a reverberatory furnace. The FeS is preferentially oxidized prior to Cu2S due to the more basic properties of iron.
The iron oxide forms a slug with silica. The molten mass is separated to form the top layer. The lower layer mostly contains copper matte (Cu2S and FeS).
Self-Reduction
The molten matte is further oxidized by air for the oxidation of the remaining iron. More silica is added which forms slag containing iron oxide.
After removing slag, the metal sulfide is oxidized by air to form Cu2O which combines with the remaining Cu2S to produce copper by self-reduction.
Cu2S + 2 Cu2O → 6 Cu + SO2
Refining of Copper by Electrolysis
The crude metal is refined by electrolysis in a bath of acidified sulfate solution using crude Cu as an anode and pure Cu as a cathode. The impurities from the anode are also used for the recovery of gold, silver, and platinum.
Chemical Compounds
In +1 or +2 states, copper forms different types of oxide, hydroxide, halides, sulfate, carbonate, nitrate, and complex compounds.
Oxidation State of Copper
Cu (II) state is the main oxidation state of copper metal with a d9 configuration. Cu (I) has the closed-shell 3d10 configuration and highly stabilizes by exchange energy.
The solid compounds in the +1 state are thermodynamically stable at moderate temperatures. Therefore, Cu2O is formed at high temperatures from CuO and CuBr2 decomposes on heating to form CuBr.
A large number of chemical compounds are formed in the +1 and +2 states of copper metal.
Cu (III) is isoelectronic with nickel (II) but only a few compounds are known in this state.
Oxides of Copper
Copper oxides exist in two forms, black cupric oxide (CuO) and red cuprous oxide (Cu2O) with oxidation states +2 and +1 respectively.
- The black color oxide (CuO) is formed during heating Cu with oxygen. The thermal decomposition of carbonate, nitrate, or hydroxide is the best way to prepare cupric oxide.
- Red Cu (I) oxide or cuprous oxide (Cu2O) is formed when alkaline solutions of Cu (II) are reduced by mild reducing agents like glucose. It occurs in nature as cuprite and is used for making ruby glass.
Copper (II) Hydroxide
The blue precipitate of copper (II) hydroxide is obtained by adding an alkali to the aqueous Cu (II) solution. The precipitate darkens on standing due to dehydration of hydroxide to oxide. It is soluble in alkali to form [Cu(OH)4]−2.
In ammonia solution, it forms deep blue [Cu(NH3)4(H2O)2]+2. The deep blue solution is called Schweizer’s reagent. It is used mostly for dissolving cellulose and making cuprammonium rayon.
Copper Halides
Only white fluoride, yellow chloride, and black bromide are known in a +2 oxidation state. Due to reduction properties, iodine reduces CuI2 to form Cu2I2.
The hydrated halides are obtained by dissolving CuCO3 or Cu(OH)2 in hydrogen halides. CuF is not well characterized.
The other halides of Cu (I) are colourless solids insoluble in water. Cuprous chloride is prepared generally by the reduction of Cu (II) with copper metal or sulfur dioxide in hydrochloric acid.
Sulfate and Nitrate
Copper sulfate (CuSO4, 5H2O) is prepared by dissolving scarp elements in dilute sulfuric acid in the presence of air and crystallizing.
A deep blue deliquescent crystalline solid, nitrate, Cu(NO3)3, 3H2O is obtained during the action of nitric acid in Cu (II) solution.
Copper (III) Compounds
Due to the larger size and higher value of the sum of the first, second, and third ionization energy, Cu (III) forms a few chemical compounds.
There are no simple examples of halides but pale green K3CuF6 may be obtained by the action of fluorine in a mixture of potassium chloride and CuCl2 at 250 °C.
Solid cuprate (III) compound like KCuO2 is formed during heating the mixture of CuO with alkali metal superoxide in the presence of an oxygen molecule.
Uses of Copper
- Copper is a good conductor of electricity and is extensively used in making wires, cables, generators, transformers, motors, and any other electrical equipment.
- It is also used for making utensils, pipes, and currency coins.
- Copper alloys like brass, bronze, constantan, manganin, and monal metal are extensively used in various domestic and industrial purposes due to their high mechanical strength and corrosion resistance.
Uses of Copper Alloys
Brass is an alloy of copper. It is obtained by alloying copper with zinc, and other metals like aluminum, nickel, and lead.
Generally, it contains 57 to 97 percent of Cu, and the remaining is Zn or other metals which modify the mechanical strength and chemical properties.
Brass is largely used in domestic equipment, statues, ship propellers, and bearings.
| Alloy of copper | |
| Alloy | Composition |
| Brass | 70% Cu and 30% Zn |
| Muntz metal | 60% Cu and 40% Zn |
| Delta metal | 55% Cu, 40% Zn and 5% Fe |
Delta metal is hard as steel and resistant to seawater. It is used for making ship propellers and bearings.
Uses of Bronze
Bronze is the alloy of copper, tin, and other metals like aluminum, manganese, and zinc, etc. The alloy bronze generally contains 4 to 25 percent of tin and other elements.
Bronze is mostly used for making statues, and currency coins. Nowadays, these alloys are gradually replaced by steel and other cheaper and lighter aluminum-based alloys.
Cupronickel
Cupronickel (10 to 30 percent nickel and the remaining copper) has high tensile strength and is resistant to chemical corrosion, rust, and acid. Therefore, cupronickel is widely used in chemical plants, telephonic systems, turbine blades, etc.