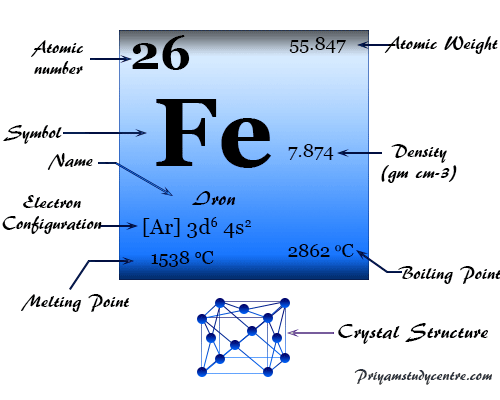
Iron element
Iron is a chemical element, silvery-white, lustrous, malleable, and ductile metal of Group 8 (VIIIB) of the periodic table with the symbol Fe and atomic number 26. It is the most useable and cheapest metal which is used by a human civilization much later than copper, silver, and gold.
In learning chemistry, the chemical element iron is included in the transition metals family due to the presence of incompletely filled d-orbitals in their atomic or ionic state.
The name comes from the old English word iren while the symbol of iron comes from the Latin word Ferrum.
Physical and chemical properties
Physical properties of iron
Iron has four allotropic crystal lattices,
- α-Fe (bcc ferromagnetic)
- β-Fe (bcc paramagnetic)
- γ-Fe (fcc paramagnetic)
- δ-Fe (bcc paramagnetic)
It is the most magnetic substance among all elements and a moderately chemically active metal in the earth’s environment.
| Iron | |||
| Symbol | Fe | ||
| Discovery | approx 3500BC | ||
| Name derived from | The Anglo-Saxon name iren and symbol of iron comes from the Latin word Ferrum | ||
| Common isotope | 26Fe56 | ||
| Oxidation states | +6, +3, +2, 0, −2 | ||
| CAS number | 7439-89-6 | ||
| Periodic properties | |||
| Atomic number | 26 | ||
| Relative atomic mass | 55.845 | ||
| Electron per cell | 2, 8, 14, 2 | ||
| Electronic Configuration | [Ar] 3d6 4s2 | ||
| Block | d-block | ||
| Group | 8 | ||
| Period | 4 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 1538 °C, 2800 °F, 1811 K | ||
| Boiling point | 2861 °C, 5182 °F, 3134 K | ||
| Molar heat capacity | 25.10 J mol−1 K−1 | ||
| Crystal structure | body-centered cubic (bcc) or face-centered cubic (fcc) | ||
| Density | 7.87 g/cm3 | ||
| Electrical resistivity | 96.1 nΩ m | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.04 Å | ||
| Covalent radius | 1.24 Å | ||
| Electronegativity | 1.83 (Pauling scale) | ||
| Electron affinity | 14.569 kJ mol−1 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 762.47 | 1561.88 | 2957.47 | |
Chemical properties of iron
The second most abundant metal, iron has the valence electronic configuration [Ar] 3d6 4s2, and common oxidation number +2 and +3.
Iron in the periodic table
Iron is placed in group 8 and period 4 in the periodic table. It is a transition metal that lies between manganese and cobalt.
History of iron
It is joined in the race of human civilization before copper, silver, and gold. It was first used by our forefathers around 4000BC. The knowledge of extracting iron from its different types of ore developed in different countries over a long interval of time.
In Egypt and Mesopotamia, the process was discovered about 2000BC. Early methods of heating ores with coal on windy sites yielded spongy metal shaped by prolonged hammering. The technique for making good quality metal was developed by using a furnace.
In modern times, smelting furnaces have become well developed. Improvement of mechanical strength by making steel from iron ores was coming in the nineteenth century.
Where is iron found?
Iron is the second most abundant metal after aluminum. It is the fourth most abundant chemical element after oxygen, silicon, and aluminum in the earth’s crust occurring to an extent of more than 5 percent or 50,000 ppm.
In the earth’s crust, the free metal is rare, terrestrial iron in basaltic rocks found in Greenland. Carbonaceous sediments are found in the United States (Missouri).
About 0.5 percent of lunar soil consists of metallic iron, which means a vast quantity of Fe is present on the moon’s surface.
Ores of iron and their formula
The most common oxide ores and minerals that occur in the earth’s crust may include,
- red hematite (Fe2O3)
- brown hematite, or limonite (2F2O3, 3H2O)
- magnetite (Fe2O3),
- siderite (FeCO3)
- pyrites (FeS2)
Pyrites cannot be economical to produce Fe and hence pyrites are not considered an ore of Fe.
The ores are found in different countries like China, Brazil, Australia, United States, and India.
1000 million tonnes of iron are produced per year all over the world. The production of crude steel after 1996 over the world is around 700 million tonnes.
Isotopes
Iron occurs in nature by four stable isotopes like 54Fe (5.845 percent), 56Fe (91.754 percent), 57Fe (2.119 percent), and 58Fe (0.282 percent).
Production process
Iron is extracted through carbon reduction in a blast furnace.
- The ore, coke, and limestone (usually ratio 8:4:1) enter the top of the furnace while a blast of preheated air at about 900 °C is brown through the holes near the bottom.
- The burning of coke produces heat near the base with a temperature near 2000 °C and falls gradually towards the top of the furnace (400 °C).
- Burning of carbon above 700 °C produces carbon monoxide which rises upward and reduces Fe2O3 and FeO. Some reduction is done by carbon.
- The limestone decomposes around 900 °C to give CaO which combines with silica to form slag (CaSiO3).
What is pig iron?
The molten slug and crude metal collect at the base of the blast furnace where they form separate layers and the slug floats on molten metal, preventing it from oxidation. These are drowned through separate holes and allowed to solidify in the sand mold to form pig or cast iron which contain impurities like carbon, silicon, phosphorus, sulfur, and manganese.
When most of the impurities present in pig-Fe are removed, the pure commercial form of metal is formed, known as wrought iron.
Interesting facts about iron
- Pure iron is chemically moderately active, silvery-white lustrous, malleable, ductile metal, and the most magnetic substance among all periodic table elements.
- The finely divided metal is pyrophoric in the air at room temperature.
- Water can reacts above 500 °C liberating hydrogen and forming Fe2O3 and FeO.
- In a heated finely divided state, it reacts with all the nonmetals (carbon, silicon, nitrogen, phosphorus, and hydrogen) to form solid metal-like compounds, Fe3C, Fe3Si, Fe3P, Fe4N, FeN, or binary ionic to covalent compounds like FeF3, FeCl3, FeS.
- The hard crystalline solid carbide Fe3C (cementite) is mainly responsible for the variation of properties of steel.
- In absence of oxygen, iron reacts with mineral acids (sulfuric acid, hydrochloric acid, or nitric acid) to give Fe (II) ion in solution.
- Dilute alkali solutions and air-free water hardly attack Fe but hot concentrated alkali hydroxides attack it.
Chemical compounds
In chemistry, iron has characteristic properties of transition metals with various oxidation states and various types of coordination and organometallic chemical compounds in different types of chemical bonding.
+2 (ferrous) and +3 (ferric) oxidation states are the main oxidation states of Fe to form a wide number of compounds in chemistry.
In Fe (II), it forms simple compounds like oxides, halides (except iodine), and other salts, together with a large number of complex compounds. FeF3, FeCl3, and FeBr3 are the halides of Fe, formed by the direct reaction of metals with respective halogens like fluorine, chlorine, and bromine.
Fe (II) is reducing in nature to form a large number of simple and complex compounds that are quite stable in +2 state. The halides, nitrate, perchlorate, and sulfate are soluble in water but the hydroxide, carbonate, phosphate, and oxalate are sparingly soluble.
It occurs in a higher oxidation state in purple potassium ferrate (KFeO4) with a +6 oxidation state, obtained by oxidation of Fe (III) by hydrochloric acid.
What is iron used for?
Iron and steel are used mainly in the different types of structural units in modern civilization and civil engineering. There are different types of steel with different properties and uses, manufactured by alloyed iron with carbon and other chemical elements.
- Mild steel is most widely used in bridges, electricity pylons, bicycle chains, cutting tools, and rifle barrels.
- It is also used as a chemical catalyst in the Haber process for the production of ammonia.
- In the small-scale production of hydrogen, it is used as a reducing agent.
- Stainless steel is an important alloy of iron that contains at least 12 to 15 percent chromium and other metals such as nickel, molybdenum, titanium, and copper which resist corrosion and the action of acids. It is widely used in architecture, bearings, cutlery, medicinal surgical instruments, and jewelry.
- Cast iron is another alloy that contains 3 to 5 percent carbon, widely used in pipes, valves, pumps, and the production of the magnet.
Function of iron in the body
It is a non-toxic essential element for all forms of life (plants and animals). A lot of iron ion is present in the hemoglobin of blood which carries oxygen from the lungs to the cell for the respiration of the body.
Deficiency of iron
The deficiency of iron in the human body causes anemia which is the most common neutralization disorder all over the world, in both developed and underdeveloped countries.
Chronic blood loss, repeated pregnancy, and hookworm infarction may cause anemia in the human body. Anemia is characterized by a lower concentration of iron in hemoglobin of blood (<12mg/liter), which causes retired growth, loss of appetite, sluggish metabolic activities, and dull or inactive attitude.