Colloid Solution
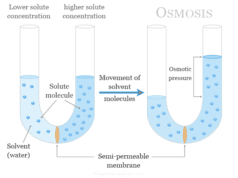
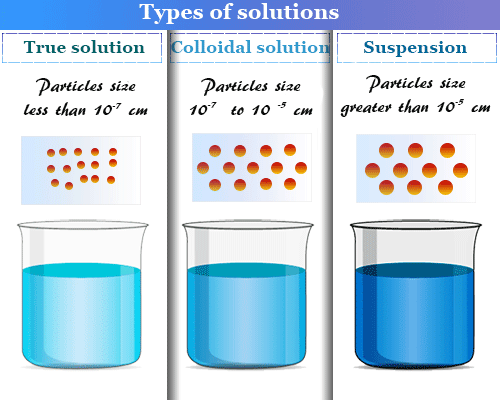
Colloid solution is a two-phase heterogeneous system in which one phase is dispersed in a fine state ranging from 1 nanometer (nm) to 1000 nanometer (nm) in another continuous or dispersion medium. The colloid system may be classified into two categories, lyophobic (solvent-hating) sols and lyophilic (solvent-loving) sols based on the affinity of the solvent. The colloidal nature of the substances is determined by the size of the colloid particles. These colloidal particles are no doubt bigger but they are not visible in the naked eye or ordinary microscope. An ultramicroscope is used to see the colloid particles. The chemical solutions are classified into three groups, true solution, colloidal solution, and suspension.
Example of Colloid Solution
- If the solid solution is dispersed in the liquid medium, it is called sols. Gold, arsenic trisulfide (As2S3), and iron(III) oxide or ferric oxide (Fe2O3) solution are examples of hydrosol since water is a continuous medium.
- Jellies, curds, and cheese are examples of gel where a liquid is dispersed in a solid medium.
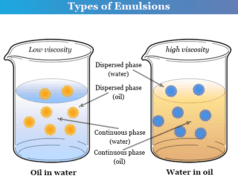
- Milk (fat in water) and oil in water are examples of emulsions where the liquid is dispersed in the liquid medium.
- When gas is dispersed in a liquid medium, it is called foam-like froth, or whipped cream.
- In aerosols like clouds or fog, the liquid and solid are dispersed in a gas medium.
- Gems ruby glass is an example of colloid where a solid is dispersed in a solid medium.
Preparation of colloids
The preparation of the colloidal system goes through three processes.
- First, the substances are directly or indirectly in the desired state of fine division in the dispersion medium.
- Next, add the protective or stabilizing agent to maintain the stability of the medium.
- Lastly, purify the colloid system from excess reagents used or by products formed by the process termed dialysis.
Preparation of Colloids by Condensation Method
The substance that is dispersed in the medium is obtained by chemical reactions or sometimes by physical changes under the controlled conditions of temperature and pressure.
Reduction Method
The gold solution may be prepared by the reduction process of chloroauric acid (HAuCl4) containing a small amount of potassium carbonate by hydrazine or formaldehyde.
Oxidation Method
The sulfur solution is easily obtained by the oxidation of an aqueous solution of hydrogen sulfide (H2S) by sulfur dioxide (SO2) in the presence of nitric acid or bromine water.
2H2S + SO2 → 3S + H2O
Double Decomposition Reaction
Silver chloride (AgCl) may be prepared by mixing a dilute solution of silver nitrate (AgNO3) and potassium chloride (KCl).
AgNO3 + KCl → AgCl + KNO3
Similarly, arsenic trisulfide (As2S3) can be prepared by passing H2S through a dilute solution of As2O3.
Hydrolysis Method
The colloid system like ferric hydroxide solution also produced by hydrolysis of ferric chloride by warm water.
FeCl3 + 3H2O → Fe(OH)3 + 3HCl
Exchange of Solvents
Sulfur solution or phosphorus solution can be prepared by dissolving them in alcohol in which they are stable and then pouring them into water.
Preparation of Colloids by Dispersion Method
The dispersion technique is a direct technique and it goes through the direct pulverizing of substance into the colloidal size and dispersing in a medium with a stabilizer.
Sometimes pulverizing is affected spontaneously. For example, when gelatine, starch, and gum are heated in water with constant stirring, the colloid sols are produced.
Pulverization also is done by electric sparking or electrolysis of metal. Colloidal solutions of gold, silver, and platinum are prepared by bringing the close electrodes of metal under-water and permit to electric discharge between them. A little specific electrolyte is added to stabilize the dispersion of the finely divided phase of the medium. It is called peptization.
For example, when a freshly precipitated Fe(OH)3 is shaken with a dilute solution of FeCl3, it produces Fe2O3 sol.
Types and Properties of Colloids
The colloid system may be classified into two categories based on the affinity of the solvent.
- Lyophobic (solvent hating) sols
- Lyophilic (solvent loving) sols
Lyophobic (Solvent Hating) Sols
Lyophobic sols like gold, and arsenic trisulfide (As2S3) have no spontaneous tendency to pass into a colloidal state and are irreversible in nature. Therefore, they are unstable, and a small amount of natural electrolyte is used for the coagulation of colloids.
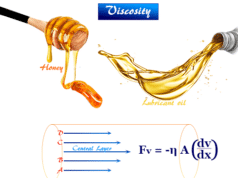
The viscosity and surface tension is almost equal to that of the medium. Particles in lyophobic sols carry either positive or negative charges. They move towards electrodes under the influence of electrical energy.
Tyndall Effect
Lyophobic sols are coloured and the colour depends on the size of the particles in suspension. For example, gold sol is red when gold particles are extremely fine but it shows blue colour when they are bigger.
The colour of the sols is due to the scattering of light by suspended particles. This is called the Tyndall effect.
Lyophilic (Solvent Loving) Sols
Lyophilic sols or solvent-loving sols like starch, and gelatine have a spontaneous tendency to pass into a colloidal state and are reversible in nature. Stable electrolytes hardly have any effect on the stability of the sols.
It has high viscosity due to a high degree of solvation and surface tension of this type of colloid system lower than that of the medium.