Osmosis and Reverse Osmosis
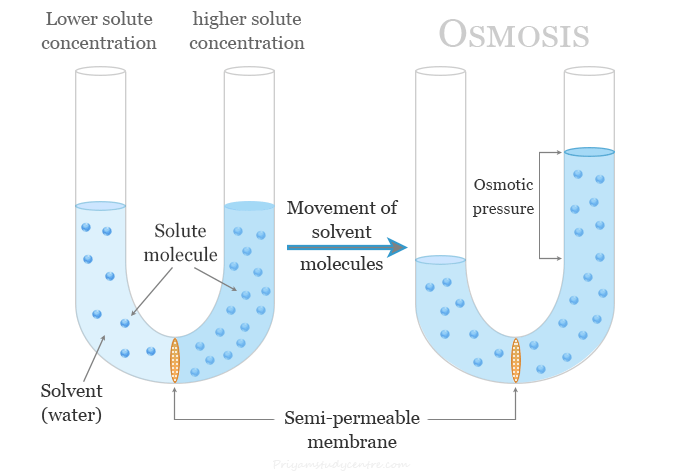
Osmosis is a natural process of movement or diffusion of solvent or water molecules through a semi-permeable membrane from a lower solute concentration region to a higher solute concentration region. When osmosis occurs in the reverse direction by applying pressure that is greater than the osmotic pressure, the process is called reverse osmosis. However, osmosis is a natural process that happens without any expenditure of energy and involves the movement of solvent until the concentrations become equal on each side of the membrane. The solvent is typically water but osmosis may occur in other liquids, supercritical liquids, or gases. In chemistry or biology laboratories, osmosis can be demonstrated by adding potato slices to a high-salt solution. The water present inside the potato moves out to the solution and the potato is to shrink.
Reverse osmosis is a water purification process or system that uses for filtering unwanted molecules and large particles such as contaminants and sediments like chlorine, salt, and dirt from drinking water.
Osmosis in Biology
It is an important process in biological systems because biological membranes are semipermeable.
In general, biological membranes are impermeable to ions, proteins, and polysaccharides molecules but permeable to non-polar or hydrophobic molecules. It may not trap small molecules like oxygen, carbon dioxide, nitrogen, and nitric oxide. The permeability of biological membranes depends on solubility, charge, and solute sizes.
Osmosis occurs in biological cells are two types endosmosis and exosmosis.
- Endosmosis: It occurs when a cell is placed in a hypotonic solution, the solvent (water) molecules move inside a cell and swell. The movement of water inside a cell is known as endosmosis.
- Exosmosis: It occurs when a cell is placed in a hypertonic solution, the solvent (water) molecules move out from the cell and the cell becomes flaccid. The movement of water outside a cell is known as exosmosis.
Osmotic Solutions
Osmosis of biological cells occurs in three different types of solutions,
- Isotonic solution: A solution that contains equal dissolved particles such as salt and other electrolytes like normal cells and blood.
- Hypertonic solution: A solution that contains more dissolved particles such as salt and other electrolytes than normal cells and blood.
- Hypotonic solution: A solution that contains fewer dissolved particles such as salt and other electrolytes than the normal cells and blood.
When an animal or a plant cell is placed in these three types of solution,
- For an isotonic solution, there will be no net movement of water across the cell membrane.
- In a hypotonic solution, the cell will gain water by osmosis.
- In a hypertonic solution, the cell will lose water due to osmotic pressure.
Example of Osmosis
It is an important process that occurs naturally in plants and animals to control several biological processes.
In Plants
- It helps to control water content inside and outside of a plant cell.
- The absorption of water from the soil by plant roots is due to osmosis. The plant roots have a higher concentration than the soil and water enters the roots by osmosis.
- It is also responsible for controlling the movement of guard cells in plants.
- It helps to maintain a concentration gradient between the root cells and the xylem vessels.
In Animal Tissues
- The red blood cells in our body are highly influenced by the osmotic pressure of our blood. The RBCs shrink in size when the blood is too dilute while they swell up or burst when the blood is concentrated.
- It regulates the flow of dissolved solids, liquids, and gases across animal cells. The semi-permeable membrane of animal cells selectively allows substances to pass in and out by osmosis. It helps to release toxic metabolic waste products such as urea from our bodies.
Diffusion and Osmosis
We may consider osmosis as a special case of diffusion that occurs through a semipermeable membrane. Only the water or other solvent molecules are moved through the membrane during the osmosis process.
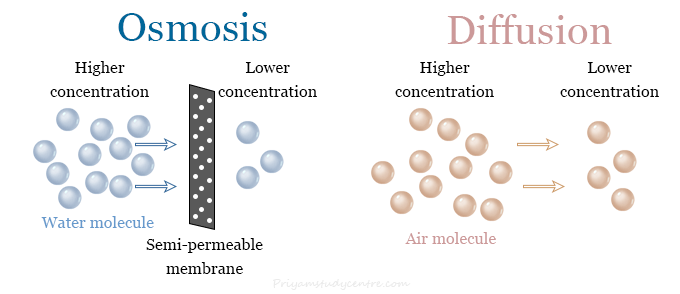
Similarities
- Osmosis and diffusion are the natural processes to equalize the concentration of two solutions.
- They are natural transport processes. Therefore, do not require any input of energy for occurring osmosis or diffusion.
- In both processes, particles are moved from a higher concentration to a lower concentration region.
Differences
- Diffusion may occur in any mixture with or without the presence of a semipermeable membrane while osmosis always occurs in the presence of a semipermeable membrane.
- Both solvent and solute particles are free to move in diffusion but only solvent or water molecules cross the membrane during osmosis.
What is Osmotic Pressure?
The osmotic pressure of a solution is the pressure required to prevent osmosis when the solution is separated from pure solvent by a semi-permeable membrane.
Osmotic Pressure Measurement
Let us take the following diagram which contains a U-tube with a semipermeable partition at the center of the tube.
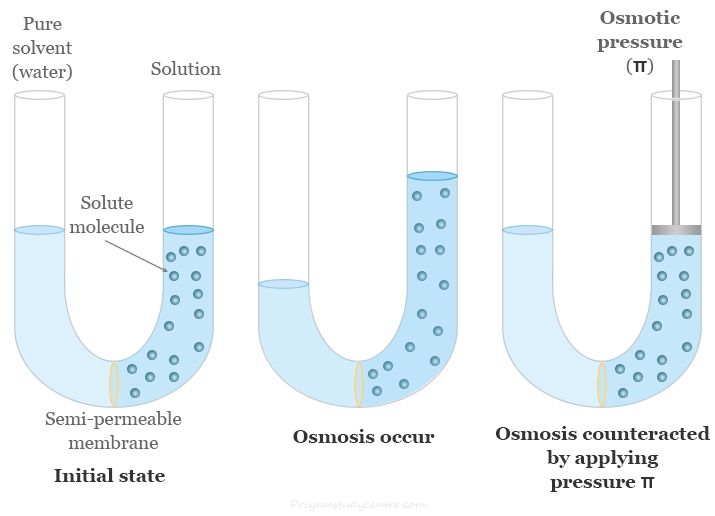
One erm of the tube is filled with a solution and the other with pure solvent. There is a piston arrangement for applying pressure on the liquids.
When osmosis occurs, the solvent passes through the semi-permeable membrane into the solution. The diffusion of the solvent into the solution through the membrane can be counteracted by applying pressure π over the solution. The excess pressure π on the solution that would just prevent osmosis is called the osmotic pressure of the solution.
Osmotic Pressure Formula
Jacobus van’t Hoff provides the quantitative formula between osmotic pressure and solute concentration. The using equation to calculate osmotic pressure,
Osmotic pressure (π) = icRT
where i = dimensionless van’t Hoff index
c = molar concentration of solute
R = ideal gas constant
T = temperature on the Kelvin scale
The osmotic pressure formula applies when the solute concentration is sufficiently low or the solution is an ideal solution.
Osmotic pressure is a colligative property. Therefore, it depends on the concentration of the solute but not on its content or chemical identity.
Significance of Osmosis
- Osmosis regulates the flow of nutrients and the release of metabolic waste products across cells.
- It controls cell to cell diffusion and maintains the mechanical structure of plant and animal cells.
- Water molecules diffuse from the soil into the root hair of plants by the osmosis process.
- It also conducts water to the upper parts of the plant through the xylem.
- Higher osmotic pressure protects the plants from drought injury.
- Osmosis is an important process that uses for the germination of seeds.