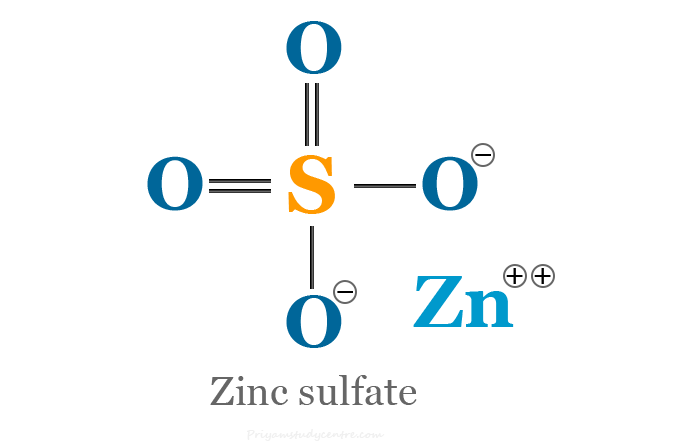
Zinc Sulfate Formula
Zinc sulfate (chemical formula ZnSO4) is an inorganic compound of zinc that is used as a dietary supplement for treating zinc deficiency. Excess supplementation of zinc sulfate causes various side effects such as abdominal pain, vomiting, headache, and tiredness. It is a combination of the chemical elements zinc and sulfur. ZnSO4 is a metal sulfate that contains Zn+2 in its structure. It formed a colorless crystal on the crystallization of ZnSO4 solution (Zn or ZnO plus dilute sulfuric acid) below 30 °C.
The heptahydrate form is the most common form of zinc sulfate that contains the chemical formula ZnSO4·7H2O. The heptahydrate form was historically called white vitriol.
Zinc is a naturally occurring mineral essential trace element for humans that uses for proper growth and development. Zinc picolinate, zinc acetate, zinc citrate, and zinc sulfate are the most common types of zinc supplements that are readily absorbed by our bodies.
Uses of Zinc Sulfate
Uses in Medicine
Zinc sulfate is an Essential Medicine according to the World Health Organization. It is needed in a basic health system. The most common medical uses of ZnSO4 are,
- Zinc sulfate supplement is used in medicine to treat a zinc deficiency.
- It is used in eye drop solutions for the treatment of eye irritation. It is an astringent for making eye drops and lotions.
- The oral form of ZnSO4 is effective for treating acne on our faces.
- It is used to supply zinc supplements in animal feeds, and toothpaste.
Uses in Fertilizer
Zinc is a micronutrient that is used for activating various enzymes for the synthesis of protein in plants. The monohydrate form of zinc sulfate is used as a fertilizer to correct the zinc deficiency in crops. It is also used for the production of various types of agricultural products.
Other Uses
- It is used for making white pigment lithophones that contain BaSO4 and ZnS. It has good covering power and is not very expensive or does not blacken by hydrogen sulfide.
- It is used for the preservation of leather.
- ZnSO4 is also used as an electrolyte for zinc electroplating to protect metals from rust or corrosion.
- It is used as a mordant in calico painting.
- Like many zinc compounds, it may also be used as an herbicide to control moss growth on roofs. The high concentration of ZnSO4 can affect the growth of moss.
Properties
The anhydrous zinc sulfate salt is a colorless crystalline solid. The hexahydrate, ZnSO4·6H2O, and heptahydrate ZnSO4·7H2O forms are also common for this salt.
All the forms are soluble in water and emit toxic fumes of zinc oxide and sulfur dioxide during decomposition. The heptahydrate form of ZnSO4 is isomorphous with MgSO4·7H2O (Epsom salt), and FeSO4·7H2O (green vitriol).
| Zinc sulfate | |
| Another name | White vitriol |
| Chemical formula | ZnSO4 (anhydrous) |
| ZnSO4·7H2O (heptahydrate) | |
| CAS number | 7733-02-0 |
| 7446-19-7 (monohydrate) | |
| 13986-24-8 (hexahydrate) | |
| 7446-20-0 (heptahydrate) | |
| Molar mass/Molecular weight | 161.47 g/mol (anhydrous) |
| 179.47 g/mol (monohydrate) | |
| 287.53 g/mol (heptahydrate) | |
| Appearance | white powder |
| Density | 3.54 g/cm3 (anhydrous) |
| Solubility | Soluble in water and alcohol |
| Melting point | 680 °C (anhydrous) |
| Boiling point | 740 °C (anhydrous) |
All forms of ZnSO4 are soluble in water to form metal aqua complex [Zn(H2O)6]2+ and SO4−2 ions. Therefore, the behavior of all forms of ZnSO4 salt in aqueous solutions is similar.
Production Process
It is found naturally in various ore in our environment, foods, and water. Industrially, it is produced by roasting the sulfide ore in air below 700 °C and leaching the mass with dilute sulfuric acid.
Zn + H2SO4 + 7 H2O → ZnSO4·7H2O + H2
The ZnSO4 salt that we use in medicine may be prepared by treating pure zinc oxide with sulfuric acid.
ZnO + H2SO4 + 6 H2O → ZnSO4·7H2O
Side Effects of Zinc Sulfate
In medicine, ZnSO4 is used by our body to prevent or treat a zinc deficiency. The powder form of ZnSO4 is an eye irritant but trace amounts are considered to be safe for humans and other animals. Excess use of zinc sulfate causes various types of side effects or allergic reactions in our bodies. The most common side effects may include,
- Nausea
- Upset stomach
- Difficulty breathing
- Swelling of your face, lips, tongue, or throat
- Dizziness and depression
- metallic taste in the mouth
Always take zinc sulfate medication after consulting your pharmacist, or other healthcare providers.
Benefits Zinc Sulfate
Zinc is an important cofactor for 70 different enzymes such as alkaline phosphatase, lactic dehydrogenase, RNA polymerase, and DNA polymerase. We use various zinc supplements to control zinc deficiency and various biological reactions.
Zinc sulfate is water-soluble and readily absorbed by our bodies. Therefore, zinc in the zinc sulfate supplement is beneficial to maintaining normal growth rates, normal skin hydration, and the senses of taste and smell.