What is Heavy Water?
Heavy water or deuterium oxide (D2O) is a form of water that contains only deuterium isotopes rather than the common hydrogen in its molecular formula. The deuterium content of water may be determined from the measurement of density, refractive index, infrared spectroscopy, or mass spectrometry. Heavy water uses widely as a moderator in nuclear power reactors, and studies reaction mechanisms and reaction kinetics in chemistry.
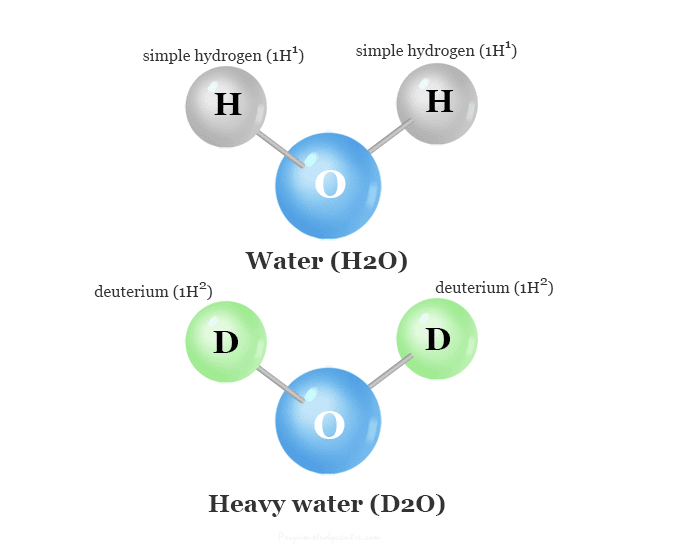
Deuterium is an isotope of hydrogen that contains a neutron and a proton in its nucleus but protium or normal hydrogen contains only one proton in its nucleus. The chemistry of heavy water is slightly different from normal water due to the presence of heavier hydrogen isotope (1H2) in the D2O molecule.
D2O Chemistry
The properties like boiling point, density, and pH value of heavy water are slightly higher than those of normal water.
| Properties | Heavy water | Light water |
| Molecular formula | D2O | H2O |
| Molar mass | 20.0276 g mol−1 | |
| Melting point | 3.82 °C | 0.0 °C |
| Boiling point | 101.4 °C | 100.0 °C |
| Density | 1.1056 | 0.9982 |
| Surface tension (N m−1) | 0.07187 | 0.07198 |
| pH | 7.44 | 7.0 |
| Refractive index at 25 °C | 1.32844 | 1.33335 |
Deuterium oxide (D2O) may be used as a starting martial for the production of various deuterium compounds such as deutero methane (CD4), deutero ammonia (ND3), deutero acetylene (C2D2), deutero sulfuric acid (D2SO4), etc
- Al4C3 + 12 D2O → 4 Al(OD)3 + 3 CD4
- Mg3N2 + 6 D2O → 3 Mg(OD)2 + 2 ND3
- CaC2 + 2 D2O → Ca(OD)2 + C2D2
- SO3 + D2O → D2SO4
Types of Heavy Water
Semiheavy Water
Semiheavy water is formed by replacing one protium (1H1) in light water with deuterium (1H2). Therefore, the chemical formula of semiheavy water is HDO.
A water sample containing an equal ratio of protium and deuterium forms 50% semiheavy water, 25% normal water, and 25% heavy water. There is a dynamic equilibrium between HDO, D2O, and H2O.
Heavy Oxygen Water
Heavy oxygen water is formed by the enrichment of heavier oxygen isotopes 17O and 18O in light water. These are commercially available and denser than normal water. It is more expensive than D2O because it is difficult to produce.
Heavy oxygen water containing 18O isotope is used for the production of fluorine-18. The isotope 18F is used in radiopharmaceuticals and radiotracers, positron emission tomography.
Tritiated Water
Tritiated water is the radioactive form of water in which protium atoms (1H1) are replaced by radioactive tritium (1H3) atoms. The chemical formula of the pure form of tritiated water or tritium oxide is T2O. Diluted tritiated water is a mixture of H2O and HTO.
Tritium is made by an artificial nuclear reaction of 3Li6 with neutrons. Lithium which uses in such transformation is taken in the form of an aluminum or magnesium alloy.
3Li6 + 1n0 → 2He4 + 1H3 (tritium)
T2O can be prepared by a reaction of tritium and oxygen in presence of a palladium catalyst or a reaction of tritium with copper oxide.
2 T2 + O2 → 2 T2O
T2 + CuO → T2O + Cu
Tritiated water may be used to measure the total volume of water in our bodies.
Heavy Water Production
Technically, deuterium is not made by any specific process. Therefore, heavy water is separated from large quantities of water containing H2O or singly deuterated water in the Girdler sulfide process.
Electrolysis of Water
D2O is made by the enrichment of ordinary water by repeated electrolysis. The overvoltage for the discharge of D+ ions is higher than that of H+ ions. Therefore, hydrogen gas evolved in the electrolysis of ordinary water. The residual water becomes gradually richer in deuterium.
Electrolysis of Alkaline Water
Heavy water is made by the repeated electrolysis of alkaline water or water containing 0.5 N sodium hydroxide (NaOH). The process is carried out for many days using a nickel electrode and a large current density till the volume goes down to one-tenth. The alkali is removed by the treatment of carbon dioxide.
The water is recovered by distillation and electrolysis is started again. 99.0 % heavy water is obtained when the volume is reduced from 28000 to 1.
Chemical exchange
The Girdler sulfide process is a method that works based on an exchange of deuterium between hydrogen sulfide and regular light water.
At present, water is enriched to 15 % deuterium content by chemical exchange. It utilizes the small differences in free energy of hydrogen and deuterium from different compounds.
HOH (l) + HSD (g) → HOD (l) + HSH
The H2O-H2S exchange may be used successfully on a large scale to produce 99 % D2O.
Argentina, Germany, the United States, India, Japan, Norway, Sweden, and Canada are the largest producers or exporters of D2O in the world.
Heavy Water Uses
- Heavy water is used in various types of nuclear reactors where it acts as a coolant or neutron moderator. As a neutron moderator, it can slow down the neutrons. Therefore, they react with the fissile uranium-235 instead of uranium-238.
- Heavy water or deuterium oxide is used for the production of deuterium. Deuterium can be prepared by the action of sodium metal on D2O or by the electrolysis of D2O containing dissolved phosphorus pentoxide.
- Heavy water or deuterium oxide is used for studying nuclear magnetic resonance (NMR) spectroscopy. Deuterium has a different magnetic moment than normal hydrogen and it does not give the 1H-NMR signals. Therefore, 1H-NMR is used to identify the position of labile hydrogens on a chemical compound because labile hydrogens are easily exchanged by deuterium atoms.
- Sometimes, it is used instead of normal water in infrared spectroscopy.
- D2O is used as a tracer to study the mechanism of various biological processes such as respiration and photosynthesis.
- Deuterium oxide help to prepare specifically labeled isotopologues of various organic compounds.
- Heavy water (D2O) is used as a source of other deuterium compounds which uses in the study of reaction mechanisms, chemical kinetics, and homogeneous catalysis.