Different Types of Alcohol
Types of alcohol uses for the manufacture of drinks or beer, vodka, and brandy include ethyl alcohol or ethanol. Alcohol is an organic compound that contains one or more hydroxyl groups. Different types of alcohols (monohydric, polyhydric, aromatic, and aliphatic) are used for the preparation of various alcoholic content like food beverages, and drugs. Alcohol is the isomers of ether or hydroxyl derivative of hydrocarbon prepared mostly from alkene with specific heat and dilute sulfuric acid under pressure. They contain carbon, hydrogen, and oxygen atoms in their structure. According to the number of functional groups (alcoholic or hydroxyl), alcohols in chemistry are classified into monohydric, dihydric, trihydric, and polyhydric. Monohydric alcohols are further classified into primary, secondary, and tertiary according to the alcoholic group attached to the alkyl.
Properties of Alcohol
The lower members of alcohol are liquids and less volatile due to association through hydrogen bonding extending over a chain of molecules. The higher members are solid and almost borderless.
Infrared spectrum studies of hydroxyl groups in alcohol show they absorbed electromagnetic spectrum radiation with a frequency from 3650 to 3580 cm−1. It is true only if there is no hydrogen bonding. Intermolecular hydrogen bonding in alcohols produces absorption in the region from 3550 to 3230 cm−1.
Solubility of Alcohol in Water
The lower members of alcohols are very soluble in water because the oxygen atom of the hydroxyl group forms hydrogen bonding with water molecules. The solubility diminishes as molecular weight increases because the hydrocarbon character of the molecule increses. Therefore, the solubility in water decreases.
However, it is not the complete story. The structure of alcohol also plays an important part in the solubility of alcohol in water. For example, n-butanol is fairly soluble in water but t-butanol is miscible in water in all proportions.
Acidic Nature of Alcohol
Alcohol liberates hydrogen by the action of strongly electropositive metals (K, Na, Mg, Al, Zn). For example, sodium or potassium reacts with ethanol to form sodium or potassium ethoxide and hydrogen.
2 C2H5OH + 2 Na → 2 C2H5O−Na+ + H2
The liberation of hydrogen atom by the action of metals shows the acidic properties of alcohols. They do not affect the pH scale of the water solution because they are weaker acids than water.
The alkoxides beings hydrolyzed by water. For this reason, ethoxide is a stronger base than the hydroxide ion.
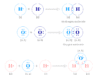
Classification of Alcohol
According to the number of functional (alcoholic or hydroxyl) groups, these organic compounds in chemistry are classified,
- Monohydric alcohol
- Dihydric alcohol
- Trihydric alcohol
- polyhydric alcohol
However, other types of classification of alcohol are given according to the attached alcoholic group to the aromatic or aliphatic compounds or hydrocarbons.
Monohydric Alcohol
Monohydric alcohols are organic compounds that contain one alcoholic or hydroxyl group.
Methanol, ethanol, propanol, isopropanol, butanol, isobutanol, and phenol are examples of such types of alcohol. There are commonly two types,
- Aromatic alcohols
- Aliphatic alcohols
Formula of Monohydric Alcohol
The monohydric alcohols are the simplest with the general molecular formula, CnH2n+2O or CnH2n+1OH, where n = number of carbon atoms in a specific alcohol molecule. For example, propanol contains 3 carbon atoms. Therefore, the molecular formula of propanol = C3H7OH.
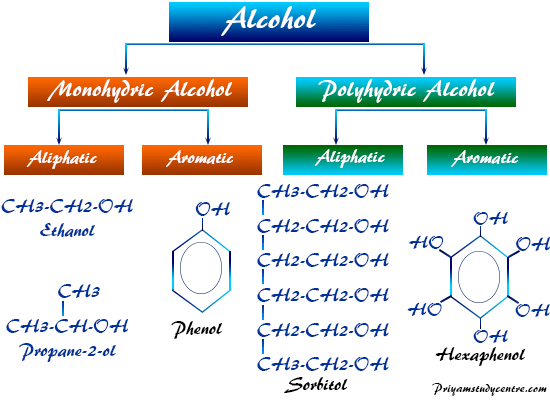
Monohydric alcohols are further classified according to the alcoholic group attached to the alkyl.
- Primary alcohol: The alcoholic group is attached to the alpha carbon atom. For example, butanol where the hydroxyl group is attached to the alpha carbon atom.
- Secondary alcohol: The alcoholic group is attached to the beta-carbon atom. For example, isopropanol where the hydroxyl group is attached to the beta carbon atom.
- Tertiary alcohol: The alcoholic group is attached to the gamma carbon atom. For example, t-butanol where the hydroxyl group is attached to the gamma carbon atom.
Polyhydric Alcohol
Dihydric and trihydric alcohol generally contain two or three alcoholic or hydroxyl groups. However, the alcohols contain four or more alcoholic groups called polyhydric alcohols. Such types of alcohol may include,
- Ethylene glycol
- Trimethylene glycol
- Pentamethylene glycol
- Isobutene glycol
Aromatic Alcohol
Aromatic alcohols are a class of organic compounds containing an alcoholic or hydroxyl group in a side chain or directly to the aromatic hydrocarbon. It may be regarded as an aryl derivative of aliphatic alcohols.
Aromatic alcohols may be classified as primary, secondary, and tertiary alcohols. The methods of preparation of this type of alcohol are similar to the aliphatic alcohols.
Types of Aromatic Alcohol
Aromatic alcohols are also classified as monohydric, dihydric, trihydric, and polyhydric phenols, according to the hydroxyl group attached to the benzene ring. Common examples of this type of alcohol are,
- Phenol
- Catechol (o-dihydroxybenzene)
- Resorcinol (m-dihydroxybenzene)
- Quinol (p-dihydroxy benzene)
Aliphatic Alcohols
Classification of alcohol is given according to the attached alcoholic group to the aromatic or aliphatic hydrocarbon. Hence these organic compounds are named aliphatic and aromatic alcohols.
Aliphatic alcohols are compounds containing hydroxyl groups in the side chain of the alkyl group.
Types of aliphatic alcohol
- Monohydric aliphatic alcohols: These chemicals contain one alcoholic group. Methanol, ethanol, propanol, isopropyl alcohol, butanol, and isobutanol are examples of monohydric alcoholic compounds.
- Dihydric aliphatic alcohols: These chemicals contain two alcoholic groups. Ethylene glycol, trimethylene glycol, pentamethylene glycol, and isobutene glycol are dihydric types of alcoholic compounds.
- Trihydric aliphatic alcohols: These chemicals contain three alcoholic groups. The only important trihydric aliphatic organic compound is glycerol or propane-1-2-2-triol. Glycerol occurs in almost all animals and vegetable oils.
- Polyhydric aliphatic alcohols: D-sorbitol, D-mannitol, and dulcitol are polyhydric aliphatic alcohols that naturally contain more than three hydroxyl groups.
Chemical Names and Formula
Different types of simpler monohydric alcohols are known by their trivial names for learning chemistry. This type of naming is obtained from the chemical derivative of the alkanes or paraffin like methane, ethane, and propane attached to the hydroxyl or alcoholic group.
| Examples of alcohol | |
| Names | Formula |
| methanol | CH3OH |
| n-propanol | CH3CH2CH2OH |
| iso-propanol | CH3CH(OH)CH3 |
| t-butanol | (CH3)3COH |
Other types of naming consider alcohol as the derivative of methanol. For example,
- CH3CH2CH3OH naming as ethyl methanol
- CH3CH2CH(OH)CH3 naming as methyl ethyl methanol
Uses of Alcohol
- They are commercially essential organic chemical compounds. They are used mostly for the production of alcoholic wine or beverages like vodka, beer, whisky, etc.
- They are used as a solvent for paints, varnishes, shellac, celluloid, and cement plants.
- When ethanol is mixed with small amounts of methanol forms methylated spirit. It is used for making automobile antifreeze mixtures and removing ink from various kinds of surfaces.
- Alcohol is further used for manufacturing dyes, antibiotics, drugs, perfumes, etc.
- Ethanol is used as an alternate fuel for the motor vehicle or energy generation (renewable energy) process.
- Phenol, methanol, and ethanol are also used as an antiseptic, alcohol-based hand sanitizer, and preparation of antibiotics drugs or bakelite.
Methods of Preparation of Alcohols
The common methods of preparation of alcohols are given below the picture,
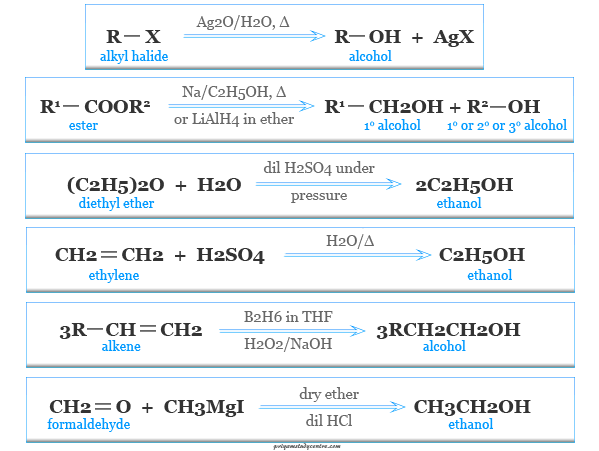
Hydrolysis of Alkyl Halides
Alcohols may be prepared during hydrolysis of an alkyl halide with silver oxide suspended in water.
RX + AgOH → ROH + AgX
Hydrolysis of Esters
Alcohols can also be prepared by hydrolysis of esters by potassium hydroxide (KOH).
R1COOR2 + KOH → R1COOK + R2OH
Hydrolysis of ester is important industrially for the preparation of alcohols that occur naturally as asters.
Bouveault Blanc reduction
Reduction of the aldehydes, ketones, or esters by reducing agent (excess sodium in ethanol or n-butanol) gives alcohols. The process is called Bouveault Blanc reduction.
- Aldehyde: RCHO → RCH2OH
- Ketone: R2CO → R2CHOH
- Ester: R1COOR2 → R1CH2OH + R2OH
Heating Ethers
it may be prepared by heating ethers with dilute sulfuric acid under pressure. For example, diethyl ether forms ethanol.
(C2H5)2O + H2O → 2 C2H5OH
Hydration of Alkenes
Alkenes may be hydrated to alcohols during absorption in concentrated sulfuric acid followed by hydrolysis of the alkyl sulfate with water.
For example, ethanol can be prepared industrially from ethylene by this method.
- Ethylene absorbs concentrated sulfuric acid at 75−80 °C under pressure to give ethyl hydrogen sulfate and ethyl sulfate.
- Hydrolysis of the mixture from the reaction with water gives ethanol. Some diethyl ether is also formed by this process.
Hydroboration Oxidation
Alkenes react rapidly with diborane (B2H6) in ether or THF at room temperature producing trialkyl boranes. Trialkyl boranes on further oxidation with alkaline hydrogen peroxide produce primary alcohols.
3 RCH=CH2 + BH3 → (RCH2−CH2−)3B
(RCH2−CH2−)3B + H2O2 → 3 RCH2CH2OH +H3BO3
Alcohols from Grignard Reagent
Primary, secondary, and tertiary alcohols may be produced from appropriate carbonyl compounds during the action of the Grignard reagent.
Yeast Fermentation Process
Fermentation in the environment is the earliest method for the preparation of ethanol by the chemical catalyst. It is used mostly for the manufacture of different types of drinks brands like beer, wine, whisky, and brandy.
- The common materials used for these chemical equilibrium reactions are wheat, barley, or potato.
- The mashed materials boil at 50 °C for one hour. The melt contains the enzyme diastase effects on starch to convert maltose and sugar by hydrolysis,
2 (C6H10O5)n + n H2O → n C12H22O11 - The liquid is cooled to 300 °C and fermented generally with yeast for 1 to 3 days. Yeast contains various enzymes, among which maltase converts maltose into glucose.
- Zymase further converted glucose into ethanol.
The carbon dioxide gas is recovered and sold as a by-product. The fermented liquor contains 6 to 10 percent alcohol used mostly for the manufacture of wine, beer, brandy, or other types of food beverages.