Zinc Metal
Zinc is a chemical element or silvery lustrous metal of Group 12 or IIB of the periodic table with atomic number 30 and symbol Zn. It is used mainly for making alloys, corrosion-resistant coating, and electrochemical cells or dry cells. Among group 12 elements of the periodic table, only mercury forms a limited number of compounds in the +1 oxidation number or state but other elements like Zn and Cd form the chemical compounds in the +2 state. The rise of shielding electrons along the 3d-orbital gradually makes the d-electron, a part of the inner core of the zinc atom. Therefore, it leaves only s-electrons for chemical bonding.
Where is Zinc Found?
It is found more abundant (76 ppm) in the earth’s crust than copper (68 ppm). Zinc blende (ZnS) and calamine (ZnCO3) are the most common ores of metal. The other minor important ores are franklinite (ZnO, Fe2O3) and willemite (Zn2SiO4).
It is found mostly in Canada, Russian countries, Australia, China, Peru, and the United States.
The making of brass from copper and zinc ores was known from ancient times in Palestine, Greece, Rome, India, and China. The extraction of the metal at this time was difficult.
The name zinc may be related to the German word Zinke meaning spike or tooth. The name also originates from the Latin word leucoma or white deposit.
Properties of Zinc
Zinc has a closed-packed hexagonal crystal lattice with elongated distances between the layers making the metal denser than those of copper and silver.
The low melting and boiling point of the metal reflects the weak participation of the outer ns electron for metallic bonding.
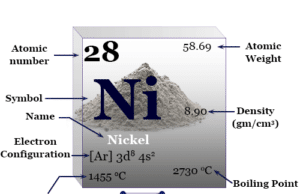
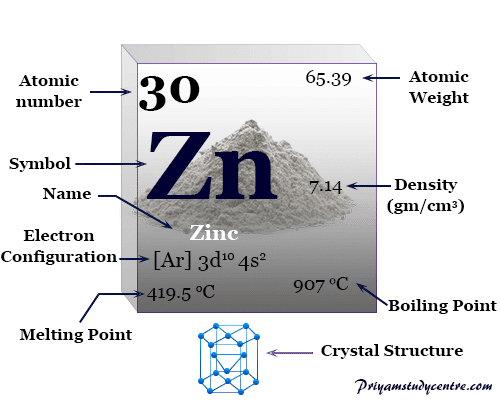
Some physical and atomic properties of zinc are given below in the table,
| Zinc | |||
| Symbol | Zn | ||
| Discovery | Andreas Marggraf | ||
| Name derived from | German word Zinke meaning spike or tooth and the Latin word leucoma or white deposit | ||
| Common isotope | 30Zn64 | ||
| Common oxidation state | +2 | ||
| CAS number | 7440-66-6 | ||
| Periodic properties | |||
| Atomic number | 30 | ||
| Relative atomic mass | 65.38 | ||
| Electron per cell | 2, 8, 18, 2 | ||
| Electronic Configuration | [Ar] 3d10 4s2 | ||
| Block | d-block | ||
| Group | 12 | ||
| Period | 4 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 419.53 °C, 787.15 °F, 692.68 K | ||
| Boiling point | 907 °C, 1665 °F, 1180 K | ||
| Molar heat capacity | 25.470 J mol−1 K−1 | ||
| Crystal structure | hexagonal close-packed (hcp) | ||
| Density | 7.134 g/cm3 | ||
| Electrical resistivity | 59.0 nΩ m | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.01 Å | ||
| Covalent radius | 1.20 Å | ||
| Electronegativity | 1.65 (Pauling scale) | ||
| Electron affinity | unknown | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 906.40 | 1733.30 | 3832.69 | |
Zinc on the Periodic Table
It is placed in group 12 and period 4 on the periodic table with group members of mercury and cadmium. Zn is the last member of the 3d-block elements.
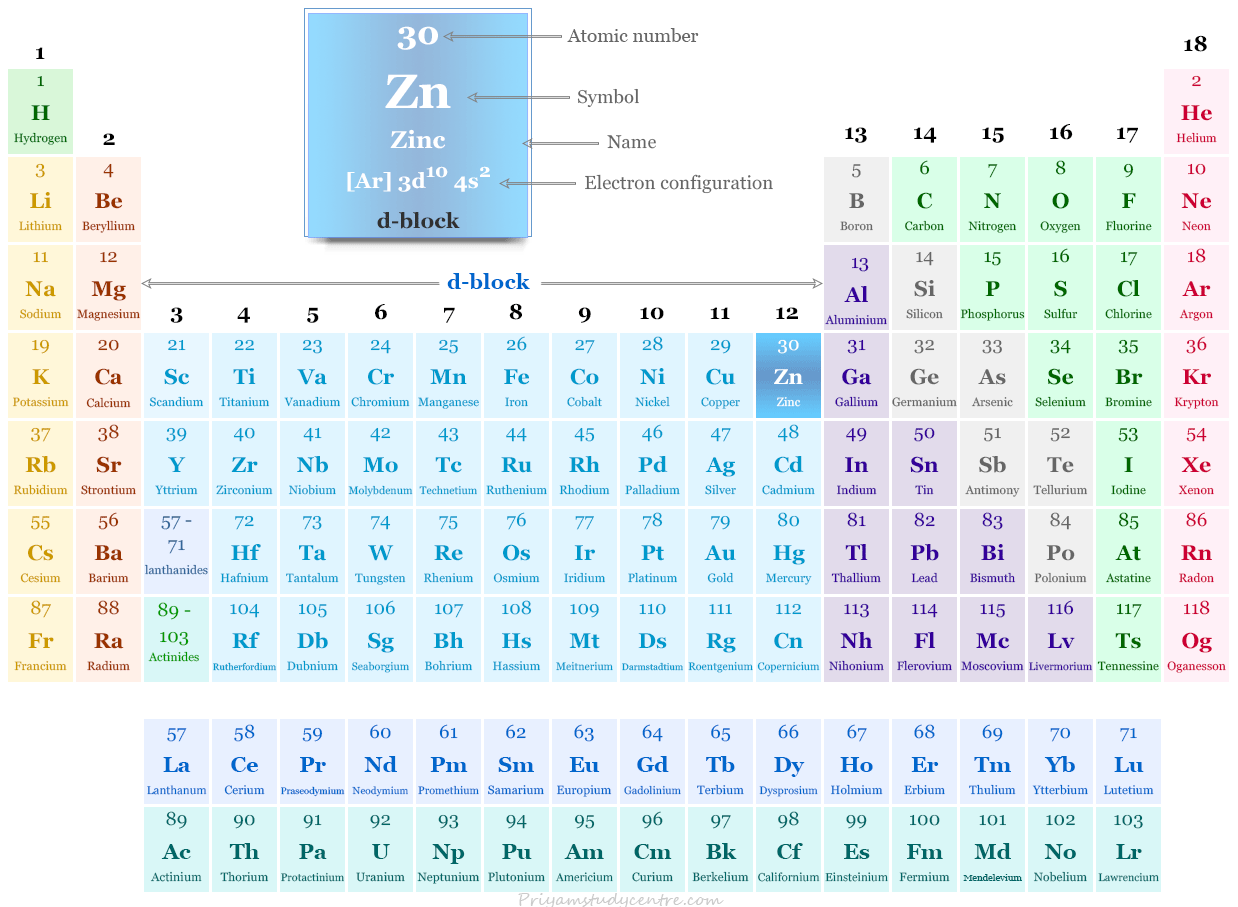
It is not a part of transition metal according to the definition of transition metals. However, due to similar properties and in order to maintain a rational classification, zinc is generally studied with transition metals.
Production of Zinc
Unlike iron, the reduction of ZnO by carbon is not an effective process for the production of zinc due to its boiling point (907 °C).
Extraction of Zinc by Electrolysis
- In the electrolysis process, the ore is roasted at a lower temperature (650 ºC) to form ZnSO4.
- The roasted mass is extracted with dilute sulfuric acid and the extract is treated with milk of lime to precipice iron, aluminum, and silica.
- Copper and cadmium are next precipitated.
- The solution of ZnSO4 electrolysis by the aluminum anode and zinc cathode under high current for the production of the pure metal.
Extraction in Blast Furnace
Presently the metal is extracted by a specially designed blast furnace. The ore is roasted to the oxide by coke which volatilizes with the hot blast. The gas is suddenly chilled by pouring molten lead on it. Therefore, the reoxidation of zinc during cooling becomes negligible.
The liquid form of zinc metal is collected at the bottom of the chamber of the furnace. It is 99 percent pure and further purified by vacuum distillation.
Facts About Zinc
- The lustrous silvery metal, zinc belongs to the 3d-block but they do not form any compound in which the d-shell is partially occupied.
- Due to poor shielding, the d-electrons pull into the inner core. The ionization energy of the zinc is also explained by the above facts.
- The third ionization energy of Zn is considerably higher than that of the first or second one indicating stronger binding for the d-electrons.
- The most stable and common oxidation state of the element zinc is +2.
- It shows certain similarities with the main group or s-block elements due to the presence of ns2 outer electronic configuration.
- The element resembles the transition metal because it forms several complex compounds with a variety of ligands like ammonia, amines, halides, and cyanides.
- However, the complexes with other strong pi-acceptor ligands like carbonyl, nitrosyl, and olefins are not known.
- The metal readily reacts with oxygen, sulfur, phosphorus, and halogens to form a variety of simple chemical compounds.
Chemical Compounds
The large difference between the 1st and 2nd ionization energies of Zn, Cd, and Hg suggests the formation of M+ ion might be possible.
In practice, the univalent state appears and is important for mercury in the form of Hg2+2. While the +2 state is favorable for Zn and Cd atoms due to the higher hydration energy of +2 ions.
Zn (II) oxides, sulfides, and halides are the most important binary compounds of the metal. It also forms unstable hydrides, nitrides, and carbides (acetylides).
What is Zinc Oxide?
Zinc oxide is the common oxide of Zn with the molecular formula ZnO. ZnO is formed by heating ZnS in the air or thermal decomposition of zinc carbonate.
It is white at ordinary temperature. ZnO turns yellow owing to the loss of oxygen from the ZnO lattice to form a non-stoichiometric composition like Zn1+xO. The vacant lattice points may trap electrons that are excited by visible electromagnetic radiation to show yellow colour.
It is amphoteric in nature and dissolves in acids to form a Zn+2 ion.
Zinc Sulfide
The sulfide of zinc (chemical formula ZnS) is very familiar to us in the routine group analysis. ZnS is produced by the direct reaction of aqueous Zn (II) with hydrogen sulfide.
ZnS appears in two crystalline solid forms like zinc blende or wurtzite. They transform each other at 1020 °C. They are readily soluble in dilute acids.
Halides
All four metal halides of zinc (ZnF2, ZnCl2, ZnBr2, and ZnI2) are known. The chloride, bromide, and iodide of Zn are also ionic with appreciable electric polarization. They crystallize as layer lattices. Fluoride like ZnF2 is ionic and highly soluble in water.
Zinc Sulfate
Zinc sulfate also called white vitriol is a colorless efflorescent crystalline solid having the chemical formula ZnSO4.
Industrially, ZnSO4 is prepared by roasting sulfide ore in air below 700 °C and leaching the mass with dilute sulfuric acid.
ZnSO4 is widely used for making calico printing and eye lotion. It is also used in medicine to supplement the intake of Zn.
Other Compounds
The crystalline form of zinc nitrate, Zn(NO3)2, 6H2O is obtained from the solution of ZnCO3 in dilute nitric acid.
Zinc acetate is another crystalline form of the element having the molecular formula Zn(CH3COO)2, 2H2O. It is obtained from the solution of ZnO in warm glacial acetic acid.
Uses of Zinc
It is largely used to provide corrosion-resistant coating in iron. A thin layer of the metal may be applied by depositing Zn on the Fe electrode (cathode) via electrolysis or by dipping it in molten zinc.
- The first process is called electrodeposition (galvanization).
- The dipping metal on molten Zn is called hot-dip galvanization.
- The metal coating is also applied by sparing zinc on other metals or by heating with Zn-powdered.
The second large use of zinc is to make different types of alloys like brass. Such alloys are used widely in making machine parts like tumbling, valves, cartridge cases, and diecasting.
The metal, Zn is used in making dry cells or batteries for torch lights, transistors, automobile, electrical, and hardware industries.
A metal compound like zinc oxide is the primary ingredient for making paints, rubber, cosmetics, pharmaceuticals, plastics, inks, soaps, and batteries.
Zinc oxide is also widely used in textile industries and medicine.