Hydrogen Sulfide (H2S) Gas
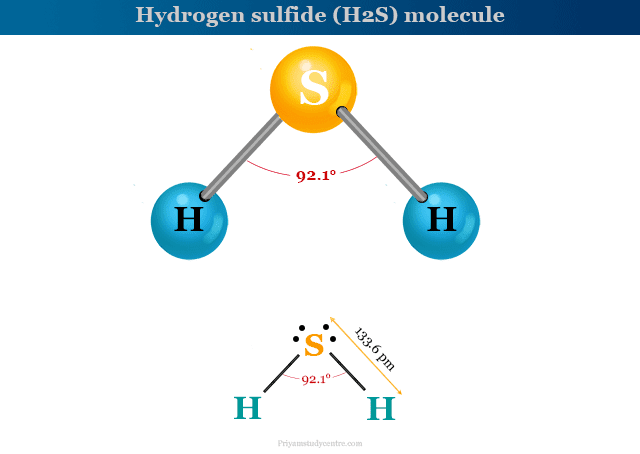
Hydrogen sulfide (chemical formula H2S) is a colorless, flammable, toxic, or poisonous gas molecule that can be detected by its smell of rotten eggs. It uses mainly for the production of sulfur and sulfuric acid. Hydrogen sulfide poisoning causes different types of health problems like eye irritation, cough, nausea, shortness of breath, etc. In 1977, Swedish chemist Carl Wilhelm Scheele first discovered and proved that H2S is a molecule that contains hydrogen and sulfur. Like carbon monoxide, hydrogen sulfide gas poisoning also prevents cellular respiration in our bodies. It is found naturally in crude petroleum, natural gas, and hot springs. It burns in oxygen with blue flame by the formation of sulfur dioxide and water. The structure of the polar H2S molecule is given below the picture,
Hydrogen Sulfide Poisoning
Hydrogen sulfide or H2S is a highly toxic gas or broad-spectrum poison for humans and animals. Therefore, it can affect the respiration or nervous system of our body.
The effects of hydrogen sulfide poisoning are comparable to carbon monoxide poisoning. Carbon monoxide binds to iron in hemoglobin to form carboxyhemoglobin complexes. Therefore, carboxyhemoglobin complexes prevent the transport of oxygen from the lungs to our cells. Similarly, H2S also binds to iron in the mitochondrial cytochrome enzymes to prevent cellular respiration in our body.
- The low explosion of H2S gas causes different types of health problems such as eye irritation, cough, nausea, shortness of breath, etc.
- The low-level long-term explosion causes the health problems such as fatigue, loss of appetite, headaches, irritability, poor memory, etc.
- A short-term high-level explosion of H2S gas affects immediately our cellular respiration which may cause death.
Hydrogen Sulfide Structure
H2S is formed by sp3 hybridization of the sulfur atom which binds with two hydrogen atoms by forming a bond angle of 92.1° and bond length 136.3 pm.
Where is Hydrogen Sulfide Found?
H2S is found naturally in crude petroleum, natural gas, and hot springs. The H2S occurring in the hot springs arises due to the hydrolysis of sulfide minerals.
Bacterial breakdown of organic materials and human wastes also produces some part of H2S in our environment. Therefore, industrial activities such as petroleum or natural gas refining, wastewater treatment, and paper mills are other sources of H2S in our environment.
How to Make Hydrogen Sulfide?
In the laboratory, hydrogen sulfide can be made by action of non-oxidizing acids such as hydrochloric acid on iron sulfide.
FeS + 2 HCl → FeCl2 + H2S
Various metal and nonmetal sulfides such as aluminum sulfide, phosphorus pentasulfide, and silicon disulfide can be liberated hydrogen sulfide gas when exposed to water.
Al2S3 + 6 H2O → 3 H2S + 2 Al(OH)3
A direct combination of hydrogen and sulfur at elevated temperatures also gives H2S. The reaction mixture is cooled to 60 °C. It may be liquefied and then purified by distillation.
H2S can be prepared by heating a mixture of solid sulfur with hydrocarbon like alkane or paraffin (CnH2n+2). It is a convenient method for the making of H2S in the laboratory.
Chemical Properties
The poisonous hydrogen sulfide (H2S) gas is slightly denser than air. It burns in oxygen to form a blue flame. Some common properties are given below the table,
| Properties | |
| IUPAC name | hydrogen sulfide |
| Chemical formula | H2S |
| Molar mass | 34.08 g mol−1 |
| Appearance | Colorless gas |
| Odor | Smell like rotten eggs |
| Density | 1.363 g dm−3 |
| Melting point | −82 °C |
| Boiling point | −60 °C |
| Solubility in water | 4 g dm−3 |
| Conjugate acid | Sulfonium |
| Conjugate base | Bisulfide |
| Dipole moment | 0.67 D |
| Heat capacity | 1.003 J K−1 g−1 |
Hydrogen Sulfide in Water
The hydrogen sulfide gas is soluble in water to form a 0.1M H2S solution. Therefore, H2S is a weak dibasic acid with pka1 = 10−7 and pka2 = 10−15. The concentration of H2S, HS−, and S− depends on the addition of acid or alkali to the saturated solution of H2S.
- The addition of H+ ions or acid to a saturated solution of H2S decreases the concentration of HS− and S−2.
- The addition of OH− ions or alkali to a saturated solution of H2S increases the concentration of HS− and S−2.
Reducing Properties
In an acid solution, the standard potential of the S/H2S couple is +0.14 volt. Therefore, H2S can be readily oxidized to sulfur by the addition of a large number of oxidants. We use potassium permanganate, potassium dichromate, and nitric acid for this oxidation.
H2S + 2 HNO3 → S + 2 NO2 + 2H2O
Precipitating Properties
Separation of metal ions becomes possible by precipitating the metal sulfides with H2S. Experiments show that the S−2 concentration in a saturated solution of H2S and 0.3 M HCl is just high enough to precipitate insoluble sulfides like HgS and CuS.
Polysulfide Formation
Polysulfides are formed when sulfur is boiled with a solution of alkali sulfide. Therefore, the solution changes its color from yellow to dark red. Disulfide (S2−2), trisulphide (S3−2) tetrasulfide (S4−2), and pentasulphide (S5−2) are common examples of polysulfide.
An acidified polysulfide solution gives free hydrides such as H2S2, H2S3, H2S4, etc. The density, viscosity, and boiling point of such hydrides commonly increase with the increasing chain length.
Hydrogen Sulfide Uses
- In the chemical industry, H2S is used mainly for the production of sulfur and sulfuric acid.
- In analytical chemistry, hydrogen sulfide is used for the qualitative analysis of metal ions. The heavy metal ions such as lead (Pb+2), copper (Cu+2), mercury (Hg+2), and arsenic (As+3) ions are precipitated from the solution in the presence of H2S.
- It is also used for the production of various inorganic sulfides which are used in various industries. Inorganic sulfides produced from hydrogen sulfide are used mainly in the leather and pharmaceutical industries.
- Hydrogen sulfide or H2S gas is used for the production of heavy water which is used widely in nuclear power reactors.