Essential Elements Nutrition
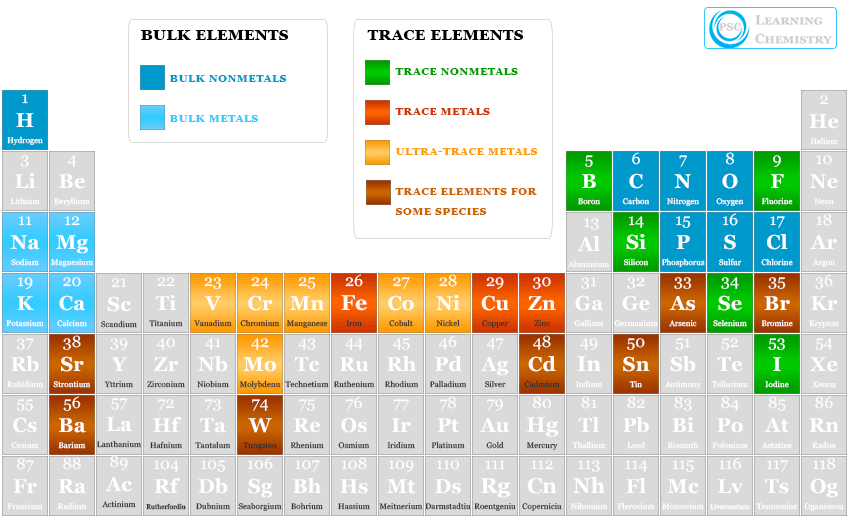
Essential element in biological science is the chemical elements that have biological functions to sustain the life of living organisms such as plants or animals. The absence or deficiency of essential or trace elements will lead to serious health problems or affect the nutrition of plants and animals. Four of these essential elements−hydrogen, oxygen, carbon, and nitrogen are required for every living system or organism. They form 99 percent of the structure of plants and animals. Phosphorus and sulfur are common examples of essential elements that are required for the structure of nucleic acids (DNA, RNA), and most amino acids and proteins. The other five bulk essential chemical elements are calcium, sodium, potassium, chlorine, and magnesium. They are involved in several biological processes. These eleven elements are constituent elements because they are required in the biological processes for relatively larger amounts.
Biological Importance of Essential Elements
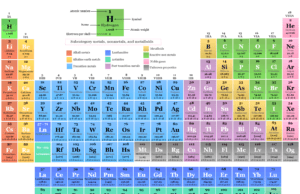
Eleven bulk essential elements like H, O, C, N, Ca, P, Na, K, S, Cl, and Mg are very important in every biological system. They are present in many living organisms and play an important role in biological reactions. The biological importance of these essential elements is given below in the table,
| Essential element | Atomic number | Biological importance |
| Hydrogen | 1 | It is the most abundant element in the biosphere. It can form hydrogen bonding which is crucial in stabilizing many biological systems. The life-supporting properties of water and many other functions like the operation of several enzymes depend on hydrogen bonding. Hydrogen can control protein folding, opening, and encapsulation of the active site in a hydrophilic and hydrophobic environment. |
| Oxygen | 8 | Oxygen is an important essential chemical element that plays a crucial role in various biochemical and physiological processes in living organisms. In the human body, it is the most abundant element after carbon, hydrogen, nitrogen, calcium, and phosphorus. It involves mainly respiration, immune function, and photosynthesis in living organisms. |
| Carbon | 6 | Carbon is the key element in any form of life. It plays a key role in the formation of organic molecules. In plants, carbon dioxide is converted to carbohydrates during photosynthesis. Respiration supplies dioxygen for the conversion of carbohydrates and other organic compounds back to carbon dioxide. |
| Nitrogen | 7 | Nitrogen is an essential constituent of several biomolecules such as proteins, enzymes, DNA, RNA, etc. Many inorganic nitrogen compounds also are involved in various biological processes. Ammonia, nitric oxide, and nitrate ions are some common example of nitrogen compounds which has a biological role. Nitrogen fixation by plants is the key reaction in the nitrogen cycle. |
| Calcium | 20 | Calcium is the major constituent of bone and teeth. It is also important in neuromuscular functions, interneuronal transmission, maintenance the cell membrane integrity, and blood coagulation. |
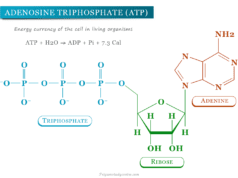
| Phosphorus | 15 | Phosphorus can create a bridge between sugar units in DNA and RNA. The phosphate group plays a crucial role during cell replication. ATP is the major source of energy in all biological processes. ATP can transfer a terminal phosphate group to glucose under the influence of the enzyme hexokinase to produce ADP and glucose 6-phosphate. The energy released in the process supplies the energy necessary for all biological processes. |
| Sodium | 11 | Sodium ions are present mostly outside the cells like blood plasma and interstitial fluid surrounding the cells. It participates in transmitting nerve signals and regulates the flow of water across the cell membranes. The transport of sugars and amino acids into cells is also influenced by sodium ions. |
| Potassium | 19 | Potassium ions occur at higher concentrations inside the cells. It can activate many enzymes and participate in the oxidation of glucose to produce ATP. Potassium ions are also involved in the transmission of nerve signals. |
| Sulfur | 16 | Sulfur is an important constituent of plant and animal bodies. Proteins contain amino acids like cysteine, cystine, and methionine involved in many important metabolic reactions. |
| Chlorine | 17 | Chlorine is the bulk essential element of our body in the form of chloride ions accompanied by sodium and potassium ions. Chloride ions are involved in the formation of HCl in gastric juice. It regulates or controls the acid-base equilibrium, fluid balance, and osmotic pressure in the biological system. Chloride ions are the major constituent of plasma and cerebrospinal fluid (CSF). It is necessary to maintain the Donnan membrane equilibrium. |
| Magnesium | 12 | The involvement of magnesium in photosynthesis is well known. It is also essential for animal physiology. Most of the magnesium present in the human body is distributed in the skeleton while others are present inside the cells. The magnesium ions in the cells are the next most important cations after potassium ions. It is important to store energy and use the same when necessary. Enzymes catalyzed replication and transcription of DNA require Zn+2 together with Mg+2. Magnesium ions are also involved in nerve impulse transmission, muscle contraction, and the metabolism of carbohydrates. |
Essential Trace Minerals
The essential trace element is the chemical element that is involved in several biological processes but the requirement of the element may be very low or moderate. Iron, copper, and zinc are the common trace elements for all living organisms.
The elements which present in exceedingly low amounts are called ultra-trace elements. Manganese, molybdenum, cobalt, nickel, and vanadium are the common ultra-trace essential elements in living systems.
Trace Elements in Minerals
The biological role of most of the trace and ultra-trace metals and nonmetals on plants and animals are given below in the table,
| Trace element | Atomic number | Biological importance |
| Boron | 5 | It is an ultra-trace element essential for healthy plants but the biochemical importance of boron is not fully understood. It is known to be involved in the synthesis of nucleic acids, metabolism of carbohydrates, hormones, and membrane formation. |
| Fluorine | 9 | Fluorine occurs mostly in bone and teeth where fluoride ion partially substituted the hydroxide ion in hydroxyapatite. It prevents the decay of tooth enamel. Excess fluoride may cause fluorosis. Excess fluoride inhibits the functions of enzymes like enolase and pyrophosphatase. |
| Silicon | 14 | Photosynthesizing diatoms form their shells from silica which are more transparent than calcium carbonate. In animals, silicon is involved in the structure of bone, cartridge, skin, and connective tissues. Silicon is concentrated in proteoglycans in the connective tissues of animals. These are complexes containing proteins and acidic chains of repeating monosaccharide residues. The deficiency of silicon can inhabits the bone growth of animals. |
| Selenium | 34 | It is an essential element but high levels may be toxic to animals. Selenium can replace sulfur in the amino acid residues of proteins and enzymes. The enzymes are effective for protecting lipids in cell membranes. It also protects the animals from carcinogenic chemicals. The element is able to bind toxic heavy metals like cadmium and mercury. |
| Iodine | 53 | Full growth of the human body contains about 20 mg of iodine. Most of which are bound to the protein thyroglobulin in the thyroid gland. Some amount of iodine is also present in the muscle, salivary gland, and ovaries. It is bound to tyrosine residues of the protein thyroglobulin and ultimately produces three thyroid hormones T3, T4, and reverse T3. The hormones influence metabolic rate, protein synthesis, carbohydrate metabolism, lipid utilization, and maintenance of the electrolyte balance. Deficiency of iodine causes mental sluggishness, lack of energy, and other health disorders. |
| Arsenic | 33 | No specific functions of arsenic are known. In mammals, it can be involved in arginine and zinc metabolism. Some marine organisms also contain arsenolipids. |
| Bromine | 35 | Bromine is essential for membrane and tissue development in animals. Bromine compounds are very common in a variety of marine organisms such as bacteria, fungi, seaweeds, and diatoms. For example, seaweeds contain the enzyme bromoperoxidase. It contains vanadium on its active site and helps to brominate some organic compounds. |
| Tin | 50 | It is an essential element involved in reproduction and growth in mammals. Tin may also form the tertiary structure of proteins |
| Iron | 26 | Iron is essential to almost all living bodies. Generally, it is a ligand in protein units. About 75% of the iron in our bodies is present in the erythrocytes in the blood which is a constituent of hemoglobin. Twenty percent is stored in non-heme iron proteins in ferritin, hemosiderin, transferrin, etc. The rest of iron in the human body is present in the myoglobin of muscle, cytochrome, xanthine, oxidase, peroxidase, etc. |
| Copper | 29 | Copper is an essential constituent of several proteins and enzymes such as ceruloplasmin, hemocyanin, cytochrome c oxidase, catalase, superoxide dismutase, ascorbic acid oxidase, etc. Therefore, Cu is involved in a number of metabolic functions in living organisms. |
| Zinc | 30 | It is an essential part of several enzymes such as carbonic anhydrase, alcohol dehydrogenase, alkaline phosphatase, carboxypeptidase, superoxide dismutase, etc. The metal is stored in the kidney, and liver in metallothionein. The prostate gland is very riched in zinc metal. The biochemical functions of zinc are based on the Lewis acid character. It has a strong preference to form tetrahedral biochemical complexes. the fact is used in stabilizing many biological structures. |
| Vanadium | 23 | Vanadium is probably an essential element for man but certainly essential for chicks and rats. The blood of some marine species like tunicates has protein-bound vanadium at a definite concentration. |
| Chromium | 24 | Chromium is primarily involved in the action of insulin in glucose metabolism. It has also a biological role in lipoprotein and the transport of amino acids through cells. |
| Manganese | 25 | Manganese is an essential constituent or cofactor of several enzymes such as arginase, pyruvate carboxylase, superoxide dismutase, and peptidase. It is also required for the growth of bone in the human body. Manganese is also involved in several biological processes such as reproduction, proper functioning of the nervous system, hemoglobin synthesis, cholesterol biosynthesis, and synthesis of mucopolysaccharides and glycoproteins. The deficiency of metals in animals affects growth, sterility, accommodation of fat in the liver, increases the activity of serum, etc. |
| Cobalt | 27 | The element cobalt is essential for growing tissues in all animals. It is the main constituent of cyanocobalamin or vitamin B12. The deficiency of cobalt in the human body owing to decreases in the production of red blood cells which causes anemia. |
| Nickel | 28 | The natural biological role of nickel was established after the discovery of metal in enzyme urease. The enzyme is present in many plants and bacteria. It catalyzes the hydrolysis of urea. |
| Molybdenum | 42 | Molybdenum is the only element in the second transition series which has natural biological functions. More than thirty enzymes in microorganisms, plants, and animals contain the metal molybdenum. Nitrogen fixation enzyme nitrogenase contains a characteristic polymetallic cluster with the iron-molybdenum cofactor. Other enzymes function as oxidases, reductases, dehydrogenases with various molybdenum cofactors. |
| Tungsten | 74 | Tungsten is the only essential element in the third transition series which has natural biological functions. Proteins containing tungsten are rare and mainly found in thermophilic anaerobes. The essential element tungsten occurs in several enzymes with molybdenum. |