Solid
Solid State of Matter
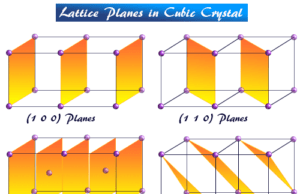
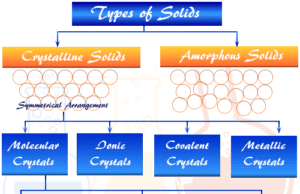
A solid state of matter in chemistry or chemical science is characterized by its high density, low compressibility, and definite structure compared with liquid, gaseous materials. The definite structure, high rigidity, and considerable mechanical strength suggest that the atoms, molecules, or ions in solids are relatively close together. Most solids are very easily converted to a liquid state by increasing temperature. Solids are classified into two broad categories, crystalline and amorphous solids. The term solid is generally employed for substances that are crystalline in nature.
Crystalline Solids Examples
They are materials whose constituents such as atoms, ions, or molecules are arranged in a definite order. The definite and ordered arrangement of constituents extends over a large distance in the crystal lattice. Crystalline solids like sodium chloride (NaCl), ice, or sugar have a sharp melting point.
Amorphous Solids Examples
Amorphous solids such as glass, pitch, rubber, or plastics have many characteristics of crystal such as definite shape, rigidity, and hardness but they do not contain regular structure and melt gradually over a range of temperatures.
In this part of online learning chemistry, we study the solid state of matter with examples, materials, structure, and shapes.