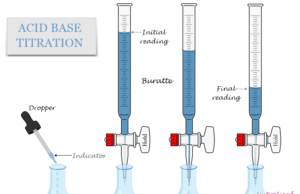
Acid Base Titration
What is Acid Base Titration?
Acid base titration in chemistry is an experimental procedure used to calculate the unknown concentration of an acid or base...
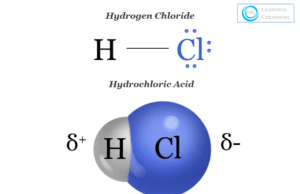
Hydrochloric Acid
Hydrochloric Acid and Hydrogen Chloride
Hydrochloric acid or muriatic acid is an aqueous solution of hydrogen chloride gas with the chemical formula HCl. It was...
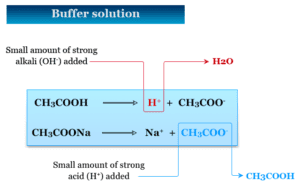
Buffer
Buffer Solution and pH
Buffer solution simply called buffer in analytical chemistry or biology is a solution whose pH remains virtually unchanged upon the addition...
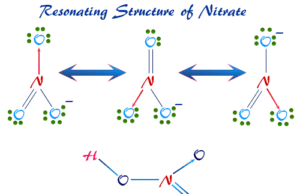
Nitric Acid
Nitric Acid (HNO3)
Nitric acid (chemical formula HNO3) is colorless, fuming, highly corrosive liquid that uses mostly for the production of fertilizers. Concentrated nitric acid...
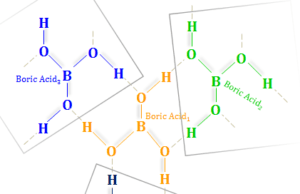
Boric Acid
Boric Acid Powder
Boric acid or Boric Powder, also called hydrogen borate or orthoboric acid is a white crystalline solid with the chemical formula of...
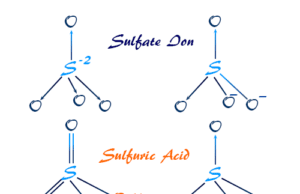
Sulfuric Acid
Sulfuric Acid Chemical Formula
Sulfuric acid, also called sulphuric acid or hydrogen sulfate (chemical formula H2SO4) is a commercially important dense, colorless, oily, corrosive liquid...
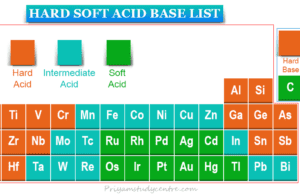
Hard Soft Acid Base
Hard Soft Acid Base List
Hard soft acid base theory or HSAB principle was proposed by Ralph Pearson in 1963. HSAB principle proposed that the...
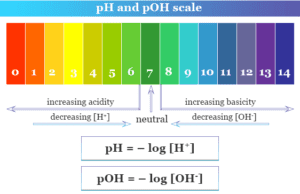
pH Scale
How to Calculate pH and pOH of a Solution?
pH and pOH scale define and measure the acidity and basicity of neutral, acidic, and basic...
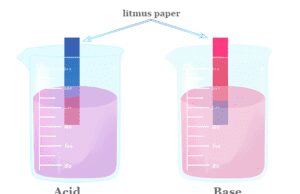
Acids Bases
Acids and Bases Properties
Acids and bases in chemistry commonly possess some opposite properties. For example, acids are chemical substances whose aqueous or water solution...
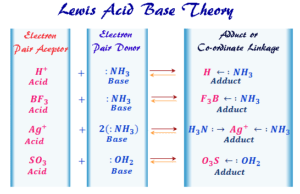
Lewis Acids Bases
Lewis Acid and Base Definition
Lewis acid base concept or theory explains the acids and bases properties in terms of electronic structure with the formation of...