Redox
Redox Reaction in Chemistry
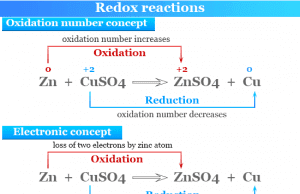
Redox or oxidation-reduction in chemistry is a type of chemical reaction where the oxidation numbers or states of reacting atoms or ions are changed. Oxidation-reduction reactions or redox reactions are also examples of electron transfer reactions where electron/electrons from one chemical species transfer to another. Oxidation is a process that results in the loss of one or more electrons by atoms or ions. Similarly, reduction is a process that results in the gain of one or more electrons by atoms or ions. Oxidation and reduction are always found to go hand in hand during a redox reaction. Whenever an element or a compound is oxidized, another compound must be reduced. The oxidation and reduction process can be explained on the basis of electron transfer and oxidation numbers.
In this category of online learning chemistry, we study the oxidation and reduction reactions or processes in chemistry.