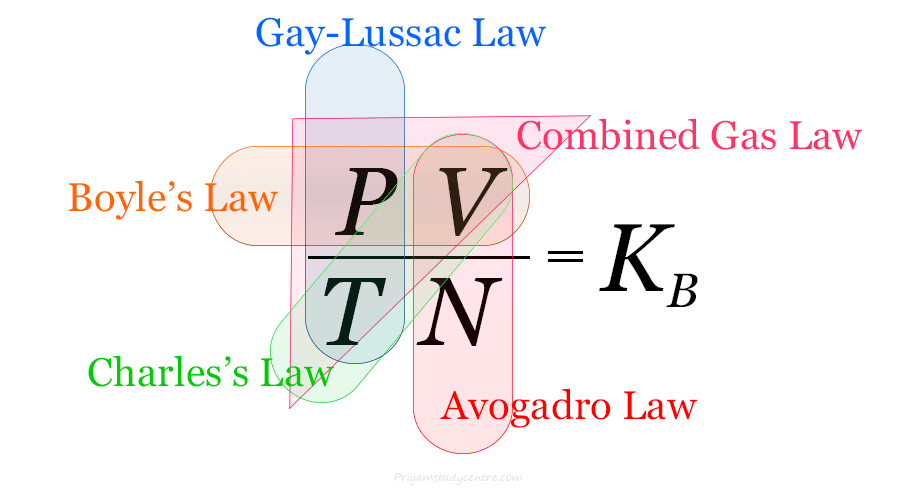Graham’s Law
Graham's Law of Diffusion
Graham's Law of diffusion and effusion in chemistry was proposed by Scottish physical chemist Thomas Graham in 1948 to study the...
Van der Waals Equation
What is Van der Waals Equation?
Van der Waals equation is given by modifying the ideal gas law in 1873. He put the reasons for...
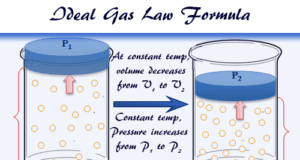
Ideal Gas Law
What is Ideal Gas Law?
Ideal gas law or perfect gas law represents the mixed relationship between pressure, volume, and temperature of gases for learning...
Kinetic Theory of Gases
What is Kinetic Theory of Gases?
Kinetic theory of gases and kinetic gas equation first-time developed by Bernoulli in 1738 to derive the molecular properties...
Ideal Gas Law Problems with Solutions
Ideal Gas Law Problems with Answers
Ideal gas law problems with solutions contain online questions and answers for 9, 10, 11, and 12 grade or...
Critical Constants
Critical constants of real gas
Critical constants like critical temperature, critical pressure, and critical volume of gas determine the condition and formula of liquefaction of...
Gases
What are gases?
Gases are the state of matter in which molecules are far apart from each other and characterized by a lack of definite...
Ideal and Real Gases
Ideal gas and real gas
Ideal gas and real gas molecule can be compared by the ideal gas law or equation for different types of gases....
Heat Capacity Gases
What is Heat Capacity?
Heat capacity (Specific) of gases is defined as the amount of heat required to raise the temperature of one gram of...