Heat Energy
Energy and Heat
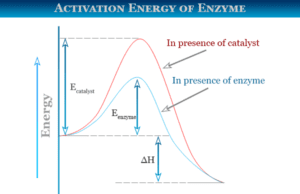
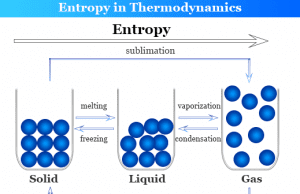
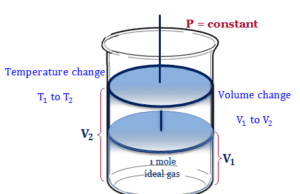
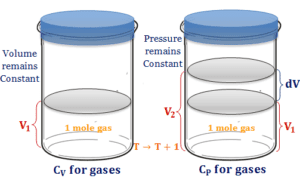
Heat energy is the form of energy that can be transferred from a higher-temperature body to a lower temperature. One object with a higher temperature loses it and others gain it. Heat transfer can be measured mostly by Joule (J) and calorie (cal). For example, heat flow increases the temperature of the colder body and decreases the temperature of the hotter body. All the other forms of energy are completely converted into work but heat can not be wholly converted into work. Any attempt to convert the whole quantity would affect a permanent change in the system. For example, a substance absorbs energy without increasing temperature changing the physical state or phase (solid, liquid, gas, or vapor) of the system by melting, sublimation, and boiling.
Heat Transfer Unit
Heat transfer can be measured by unit Joule (J) in the SI system and Calorie (cal) in the CGS system. It is also measured by unit kilocalorie (kcal) and British thermal unit (BTU).
- Joule (J): It is defined as the amount of energy needed to raise the temperature of a given mass by one degree.
- Calorie (cal): It is defined as the amount of energy needed to raise the temperature of one gram of clean water by one degree Celsius.
In this category of online learning chemistry, we study meaning, formula, examples, and unit heat energy.