Ethylene
Ethylene Gas
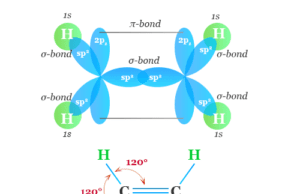
Ethylene is a simple organic compound or unsaturated hydrocarbon that contains a double bond with the molecular formula C2H4 or CH2=CH2. It may be...
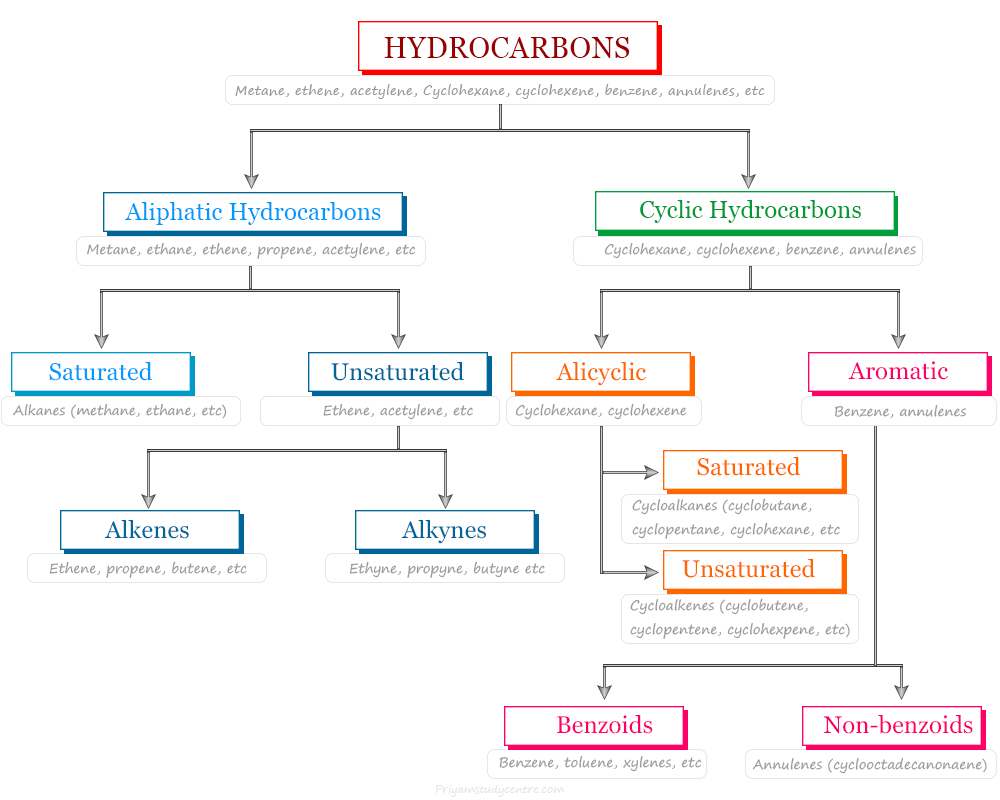
Alkenes
Alkenes in Organic Chemistry
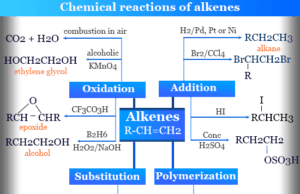
Alkenes or olefins in organic chemistry are unsaturated hydrocarbon or organic compounds that contain at least one double bond along with...
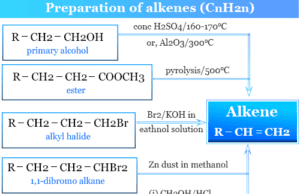
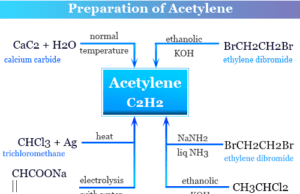
Acetylene
Acetylene Gas
Acetylene or ethyne is a colorless gas with the general chemical formula C2H2. When acetylene gas combustion with oxygen it forms a luminous...
Methane Gas
Sources of Methane Gas
Methane gas (chemical formula CH4) also called marsh gas is the simplest hydrocarbon compound of the alkanes or paraffin with a...
Alkenes Olefins
Alkene or Olefin in Organic Chemistry
Alkenes names as olefins like ethylene, propylene, butylene, etc are unsaturated hydrocarbon containing at least one double or olefinic...
Alkenes Properties
Physical Properties of Alkenes
Physical properties of alkenes and alkanes in organic chemistry are very similar. Alkenes are unsaturated hydrocarbons but alkanes are saturated hydrocarbons. Due to...
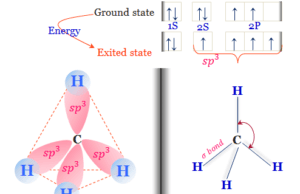
Alkanes
Structure of Alkanes
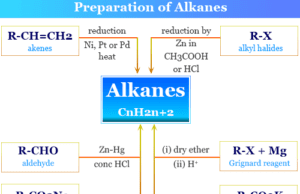
Alkanes (Paraffin) in organic chemistry are saturated hydrocarbons or organic compounds having the general molecular formula CnH2n+2. They are mainly two types,...